Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peripheral Protein Unfolding Drives Membrane Bending
Langmuir ( IF 3.7 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1021/acs.langmuir.8b01136 Hew Ming Helen Siaw 1 , Gokul Raghunath 1 , R. Brian Dyer 1
Langmuir ( IF 3.7 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1021/acs.langmuir.8b01136 Hew Ming Helen Siaw 1 , Gokul Raghunath 1 , R. Brian Dyer 1
Affiliation
|
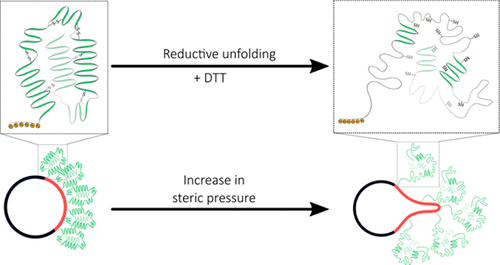
Dynamic modulation of lipid membrane curvature can be achieved by a number of peripheral protein binding mechanisms such as hydrophobic insertion of amphipathic helices and membrane scaffolding. Recently, an alternative mechanism was proposed in which crowding of peripherally bound proteins induces membrane curvature through steric pressure generated by lateral collisions. This effect was enhanced using intrinsically disordered proteins that possess high hydrodynamic radii, prompting us to explore whether membrane bending can be triggered by the folding–unfolding transition of surface-bound proteins. We utilized histidine-tagged human serum albumin bound to Ni-NTA-DGS containing liposomes as our model system to test this hypothesis. We found that reduction of the disulfide bonds in the protein resulted in unfolding of HSA, which subsequently led to membrane tubule formation. The frequency of tubule formation was found to be significantly higher when the proteins were unfolded while being localized to a phase-separated domain as opposed to randomly distributed in fluid phase liposomes, indicating that the steric pressure generated from protein unfolding can drive membrane deformation. Our results are critical for the design of peripheral membrane protein-immobilization strategies and open new avenues for exploring mechanisms of membrane bending driven by conformational changes of peripheral membrane proteins.
中文翻译:

周围蛋白的展开驱动膜弯曲
脂质膜曲率的动态调节可以通过许多外围蛋白结合机制来实现,例如两亲性螺旋的疏水插入和膜支架。最近,提出了另一种机制,其中外围结合蛋白的拥挤通过横向碰撞产生的空间压力引起膜曲率。使用具有高流体动力学半径的内在无序蛋白可以增强这种作用,促使我们探索是否可以通过表面结合蛋白的折叠-展开转变来触发膜弯曲。我们利用组氨酸标记的人血清白蛋白与包含脂质体的Ni-NTA-DGS结合作为我们的模型系统,以检验这一假设。我们发现蛋白质中二硫键的减少导致HSA的展开,随后导致膜小管形成。发现当蛋白质在定位于相分离域的同时展开时,肾小管形成的频率显着更高,这与随机分布在液相脂质体中相反,这表明由蛋白质展开产生的空间压力可以驱动膜变形。我们的结果对于外周膜蛋白固定策略的设计至关重要,并为探索由外周膜蛋白构象变化驱动的膜弯曲机制开辟了新途径。表明蛋白质解折叠产生的空间压力可以驱动膜变形。我们的结果对于外周膜蛋白固定策略的设计至关重要,并为探索由外周膜蛋白构象变化驱动的膜弯曲机制开辟了新途径。表明蛋白质解折叠产生的空间压力可以驱动膜变形。我们的结果对于外周膜蛋白固定策略的设计至关重要,并为探索由外周膜蛋白构象变化驱动的膜弯曲机制开辟了新途径。
更新日期:2018-06-20
中文翻译:

周围蛋白的展开驱动膜弯曲
脂质膜曲率的动态调节可以通过许多外围蛋白结合机制来实现,例如两亲性螺旋的疏水插入和膜支架。最近,提出了另一种机制,其中外围结合蛋白的拥挤通过横向碰撞产生的空间压力引起膜曲率。使用具有高流体动力学半径的内在无序蛋白可以增强这种作用,促使我们探索是否可以通过表面结合蛋白的折叠-展开转变来触发膜弯曲。我们利用组氨酸标记的人血清白蛋白与包含脂质体的Ni-NTA-DGS结合作为我们的模型系统,以检验这一假设。我们发现蛋白质中二硫键的减少导致HSA的展开,随后导致膜小管形成。发现当蛋白质在定位于相分离域的同时展开时,肾小管形成的频率显着更高,这与随机分布在液相脂质体中相反,这表明由蛋白质展开产生的空间压力可以驱动膜变形。我们的结果对于外周膜蛋白固定策略的设计至关重要,并为探索由外周膜蛋白构象变化驱动的膜弯曲机制开辟了新途径。表明蛋白质解折叠产生的空间压力可以驱动膜变形。我们的结果对于外周膜蛋白固定策略的设计至关重要,并为探索由外周膜蛋白构象变化驱动的膜弯曲机制开辟了新途径。表明蛋白质解折叠产生的空间压力可以驱动膜变形。我们的结果对于外周膜蛋白固定策略的设计至关重要,并为探索由外周膜蛋白构象变化驱动的膜弯曲机制开辟了新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号