当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular metal–ligand electron transfer triggered by co-ligand substitution†
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8dt01234b Alexandra Ziesak 1, 2, 3, 4 , Lena Steuer 1, 2, 3, 4 , Elisabeth Kaifer 1, 2, 3, 4 , Norbert Wagner 4, 5, 6, 7 , Johannes Beck 4, 5, 6, 7 , Hubert Wadepohl 1, 2, 3, 4 , Hans-Jörg Himmel 1, 2, 3, 4
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8dt01234b Alexandra Ziesak 1, 2, 3, 4 , Lena Steuer 1, 2, 3, 4 , Elisabeth Kaifer 1, 2, 3, 4 , Norbert Wagner 4, 5, 6, 7 , Johannes Beck 4, 5, 6, 7 , Hubert Wadepohl 1, 2, 3, 4 , Hans-Jörg Himmel 1, 2, 3, 4
Affiliation
|
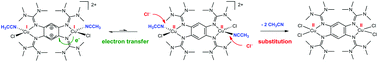
The possibility of directed stimulation of intramolecular electron transfer between a metal and a redox-active ligand in a molecular coordination compound is the key to its application in molecular catalysis and other research themes. Although the stimulation by a substitution reaction of the co-ligands is often postulated as key step in catalytic cycles using redox-active ligands as electron reservoirs, there are only a few explicit examples for such reactions. Herein we report the synthesis of the first dicationic and dinuclear CuI complexes featuring the oxidized form of a redox-active tetrakisguanidine ligand (1,2,4,5-tetrakis(tetramethylguanidino)benzene 1) as a bridging ligand and two neutral co-ligands L (acetonitrile or pyridine), [1{Cu(Cl)L}2]2+. An intramolecular electron transfer between the copper atom and the tetrakisguanidine ligand 1, leading to a dinuclear CuII complex with the reduced form of the tetrakisguanidine ligand 1, is triggered by substitution of the neutral co-ligands L.
中文翻译:

共配体取代引发的分子内金属-配体电子转移†
在分子配位化合物中直接刺激金属与氧化还原活性配体之间的分子内电子转移的可能性是其在分子催化和其他研究主题中应用的关键。尽管通常假设通过使用氧化还原活性配体作为电子贮库的催化循环中的关键步骤来促进共配体的取代反应,但此类反应的例子很少。本文我们报告的第一双阳离子的合成和双核铜我络合物设有一个氧化还原活性tetrakisguanidine配体的氧化形式(1,2,4,5-四(四甲基胍基)苯1),为桥连配体和两个中性共配体L(乙腈或吡啶),[ 1 {Cu(Cl)L}2 ] 2+。铜原子和配体tetrakisguanidine之间形成分子内电子转移1,导致双核铜II络合物与配体tetrakisguanidine的还原形式1中,通过中性的取代触发共配体L.
更新日期:2018-06-21
中文翻译:

共配体取代引发的分子内金属-配体电子转移†
在分子配位化合物中直接刺激金属与氧化还原活性配体之间的分子内电子转移的可能性是其在分子催化和其他研究主题中应用的关键。尽管通常假设通过使用氧化还原活性配体作为电子贮库的催化循环中的关键步骤来促进共配体的取代反应,但此类反应的例子很少。本文我们报告的第一双阳离子的合成和双核铜我络合物设有一个氧化还原活性tetrakisguanidine配体的氧化形式(1,2,4,5-四(四甲基胍基)苯1),为桥连配体和两个中性共配体L(乙腈或吡啶),[ 1 {Cu(Cl)L}2 ] 2+。铜原子和配体tetrakisguanidine之间形成分子内电子转移1,导致双核铜II络合物与配体tetrakisguanidine的还原形式1中,通过中性的取代触发共配体L.











































 京公网安备 11010802027423号
京公网安备 11010802027423号