当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Tandem Reaction of Quinazolinone‐Based Nitriles with Arylboronic Acids: Synthesis of 2‐(4‐Arylquinazolin‐2‐yl)anilines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-07-05 , DOI: 10.1002/adsc.201800615 Yetong Zhang 1 , Yinlin Shao 1 , Julin Gong 1 , Kun Hu 1 , Tianxing Cheng 1 , Jiuxi Chen 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-07-05 , DOI: 10.1002/adsc.201800615 Yetong Zhang 1 , Yinlin Shao 1 , Julin Gong 1 , Kun Hu 1 , Tianxing Cheng 1 , Jiuxi Chen 1
Affiliation
|
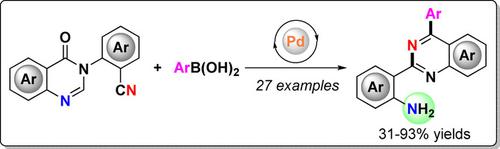
A palladium‐catalyzed tandem reaction of 2‐(quinazolinone‐3(4H)‐yl)benzonitriles with arylboronic acids has been developed, allowing access to a new class of 2‐(4‐arylquinazolin‐2‐yl)anilines that were often difficult to prepare using previous methods. In particular, the newly produced amino group is amenable to further synthetic elaborations, thereby broadening the diversity of the products. The structure of the newly synthesized 2‐(4‐arylquinazolin‐2‐yl)anilines was unambiguously confirmed by X‐ray crystallography. Moreover, a possible mechanism for the formation of 2‐(4‐arylquinazolin‐2‐yl)anilines is discussed.
中文翻译:

喹唑啉酮基腈与芳基硼酸的钯催化串联反应:2-(4-芳基喹唑啉-2-基)苯胺的合成
已开发了2-(喹唑啉酮-3(4 H)-基)苄腈与芳基硼酸的钯催化串联反应,使人们能够获得经常使用的新型2-(4-芳基喹唑啉-2-基)苯胺使用以前的方法很难准备。特别地,新产生的氨基适合于进一步的合成修饰,从而扩大了产物的多样性。X射线晶体学明确证实了新合成的2-(4-芳基喹唑啉-2-基)苯胺的结构。此外,讨论了形成2-(4-芳基喹唑啉-2-基)苯胺的可能机理。
更新日期:2018-07-05
中文翻译:

喹唑啉酮基腈与芳基硼酸的钯催化串联反应:2-(4-芳基喹唑啉-2-基)苯胺的合成
已开发了2-(喹唑啉酮-3(4 H)-基)苄腈与芳基硼酸的钯催化串联反应,使人们能够获得经常使用的新型2-(4-芳基喹唑啉-2-基)苯胺使用以前的方法很难准备。特别地,新产生的氨基适合于进一步的合成修饰,从而扩大了产物的多样性。X射线晶体学明确证实了新合成的2-(4-芳基喹唑啉-2-基)苯胺的结构。此外,讨论了形成2-(4-芳基喹唑啉-2-基)苯胺的可能机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号