当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Swapping Interface Contacts in the Homodimeric tRNA‐Guanine Transglycosylase: An Option for Functional Regulation
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-16 , DOI: 10.1002/anie.201804627 Frederik Rainer Ehrmann 1 , Jorna Kalim 2 , Toni Pfaffeneder 2 , Bruno Bernet 2 , Christoph Hohn 2 , Elisabeth Schäfer 2 , Thomas Botzanowski 3 , Sarah Cianférani 3 , Andreas Heine 1 , Klaus Reuter 1 , François Diederich 2 , Gerhard Klebe 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-16 , DOI: 10.1002/anie.201804627 Frederik Rainer Ehrmann 1 , Jorna Kalim 2 , Toni Pfaffeneder 2 , Bruno Bernet 2 , Christoph Hohn 2 , Elisabeth Schäfer 2 , Thomas Botzanowski 3 , Sarah Cianférani 3 , Andreas Heine 1 , Klaus Reuter 1 , François Diederich 2 , Gerhard Klebe 1
Affiliation
|
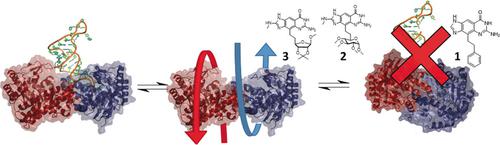
The enzyme tRNA‐guanine transglycosylase, a target to fight Shigellosis, recognizes tRNA only as a homodimer and performs full nucleobase exchange at the wobble position. Active‐site inhibitors block the enzyme function by competitively replacing tRNA. In solution, the wild‐type homodimer dissociates only marginally, whereas mutated variants show substantial monomerization in solution. Surprisingly, one inhibitor transforms the protein into a twisted state, whereby one monomer unit rotates by approximately 130°. In this altered geometry, the enzyme is no longer capable of binding and processing tRNA. Three sugar‐type inhibitors have been designed and synthesized, which bind to the protein in either the functionally competent or twisted inactive state. They crystallize with the enzyme side‐by‐side under identical conditions from the same crystallization well. Possibly, the twisted inactive form corresponds to a resting state of the enzyme, important for its functional regulation.
中文翻译:

交换同型二聚体tRNA-鸟嘌呤转糖基酶中的界面接触:功能调节的一种选择。
tRNA-鸟嘌呤转糖基酶是对抗志贺氏菌病的靶标,仅将tRNA识别为同型二聚体,并在摆动位置进行完整的核碱基交换。活性位抑制剂通过竞争性替代tRNA来阻断酶的功能。在溶液中,野生型同型二聚体仅少量解离,而突变的变体在溶液中显示出大量单体化。令人惊讶地,一种抑制剂将蛋白质转变为扭曲状态,由此一个单体单元旋转大约130°。在这种改变的几何形状中,该酶不再能够结合和加工tRNA。已经设计并合成了三种糖类抑制剂,它们以功能有效或扭曲的失活状态与蛋白质结合。它们与酶在相同条件下从同一结晶孔并排结晶。扭曲的无活性形式可能对应于酶的静止状态,这对于其功能调节很重要。
更新日期:2018-07-16
中文翻译:

交换同型二聚体tRNA-鸟嘌呤转糖基酶中的界面接触:功能调节的一种选择。
tRNA-鸟嘌呤转糖基酶是对抗志贺氏菌病的靶标,仅将tRNA识别为同型二聚体,并在摆动位置进行完整的核碱基交换。活性位抑制剂通过竞争性替代tRNA来阻断酶的功能。在溶液中,野生型同型二聚体仅少量解离,而突变的变体在溶液中显示出大量单体化。令人惊讶地,一种抑制剂将蛋白质转变为扭曲状态,由此一个单体单元旋转大约130°。在这种改变的几何形状中,该酶不再能够结合和加工tRNA。已经设计并合成了三种糖类抑制剂,它们以功能有效或扭曲的失活状态与蛋白质结合。它们与酶在相同条件下从同一结晶孔并排结晶。扭曲的无活性形式可能对应于酶的静止状态,这对于其功能调节很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号