当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ambient Electrosynthesis of Ammonia: Electrode Porosity and Composition Engineering
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-09 , DOI: 10.1002/anie.201805514 Hong Wang 1 , Lu Wang 2, 3 , Qiang Wang 4 , Shuyang Ye 2 , Wei Sun 2 , Yue Shao 1 , Zhiping Jiang 1 , Qiao Qiao 5, 6 , Yimei Zhu 6 , Pengfei Song 7 , Debao Li 4 , Le He 3 , Xiaohong Zhang 3 , Jiayin Yuan 8 , Tom Wu 9 , Geoffrey A. Ozin 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-09 , DOI: 10.1002/anie.201805514 Hong Wang 1 , Lu Wang 2, 3 , Qiang Wang 4 , Shuyang Ye 2 , Wei Sun 2 , Yue Shao 1 , Zhiping Jiang 1 , Qiao Qiao 5, 6 , Yimei Zhu 6 , Pengfei Song 7 , Debao Li 4 , Le He 3 , Xiaohong Zhang 3 , Jiayin Yuan 8 , Tom Wu 9 , Geoffrey A. Ozin 2
Affiliation
|
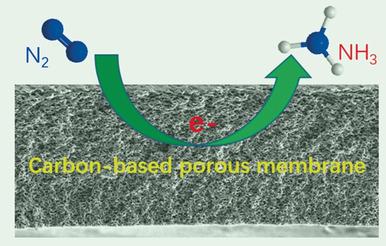
Ammonia, a key precursor for fertilizer production, convenient hydrogen carrier, and emerging clean fuel, plays a pivotal role in sustaining life on Earth. Currently, the main route for NH3 synthesis is by the heterogeneous catalytic Haber–Bosch process (N2+3 H2→2 NH3), which proceeds under extreme conditions of temperature and pressure with a very large carbon footprint. Herein we report that a pristine nitrogen‐doped nanoporous graphitic carbon membrane (NCM) can electrochemically convert N2 into NH3 in an acidic aqueous solution under ambient conditions. The Faradaic efficiency and rate of production of NH3 on the NCM electrode reach 5.2 % and 0.08 g m−2 h−1, respectively. Functionalization of the NCM with Au nanoparticles dramatically enhances these performance metrics to 22 % and 0.36 g m−2 h−1, respectively. As this system offers the potential to be scaled to industrial levels it is highly likely that it might displace the century‐old Haber–Bosch process.
中文翻译:

氨的环境电合成:电极孔隙率和组成工程
氨是肥料生产的重要前体,便捷的氢载体和新兴的清洁燃料,在维持地球生命方面发挥着举足轻重的作用。目前,合成NH 3的主要途径是通过非均相催化Haber-Bosch过程(N 2 +3 H 2 →2 NH 3),该过程在极端温度和压力条件下进行,且碳足迹非常大。在这里,我们报道原始的氮掺杂纳米多孔石墨碳膜(NCM)可以在环境条件下,在酸性水溶液中将N 2电化学转化为NH 3。NCM电极上的法拉第效率和NH 3的产生率分别达到5.2%和0.08 g m-2 h -1。用Au纳米颗粒对NCM进行功能化可将这些性能指标分别显着提高至22%和0.36 g m -2 h -1。由于该系统具有可扩展至工业水平的潜力,因此很有可能取代具有百年历史的Haber-Bosch工艺。
更新日期:2018-07-09
中文翻译:

氨的环境电合成:电极孔隙率和组成工程
氨是肥料生产的重要前体,便捷的氢载体和新兴的清洁燃料,在维持地球生命方面发挥着举足轻重的作用。目前,合成NH 3的主要途径是通过非均相催化Haber-Bosch过程(N 2 +3 H 2 →2 NH 3),该过程在极端温度和压力条件下进行,且碳足迹非常大。在这里,我们报道原始的氮掺杂纳米多孔石墨碳膜(NCM)可以在环境条件下,在酸性水溶液中将N 2电化学转化为NH 3。NCM电极上的法拉第效率和NH 3的产生率分别达到5.2%和0.08 g m-2 h -1。用Au纳米颗粒对NCM进行功能化可将这些性能指标分别显着提高至22%和0.36 g m -2 h -1。由于该系统具有可扩展至工业水平的潜力,因此很有可能取代具有百年历史的Haber-Bosch工艺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号