当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Deprotonation Approach to the Unprecedented Amino‐Trimethylenemethane Chemistry: Regio‐, Diastereo‐, and Enantioselective Synthesis of Complex Amino Cycles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-18 , DOI: 10.1002/anie.201805876 Barry M. Trost 1 , Youliang Wang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-18 , DOI: 10.1002/anie.201805876 Barry M. Trost 1 , Youliang Wang 1
Affiliation
|
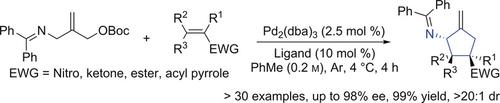
The first realization of the amino‐trimethylenemethane chemistry is reported using a deprotonation strategy to simplify the synthesis of the amino‐trimethylenemethane donor in two steps from commercial and inexpensive materials. A broad scope of cycloaddition acceptors (seven different classes) participated in the chemistry, chemo‐, regio‐, diastereo‐, and enantioselectively generating various types of highly valuable complex amino cycles. Multiple derivatization reactions that further elaborated the initial amino cycles were performed without isolation of the crude product. Ultimately, we applied the amino‐trimethylenemethane chemistry to synthesize a potential pharmaceutical in 8 linear steps and 7.5 % overall yield, which previously was achieved in 18 linear steps and 0.6 % overall yield.
中文翻译:

一种前所未有的氨基-三甲基甲烷化学的去质子化方法:复杂氨基循环的区域,非对映和对映选择性合成
报道了使用脱质子化策略简化氨基-三亚甲基甲烷供体的合成过程,这是从商业和廉价的材料分两个步骤进行的,首次实现了氨基-三甲甲烷化学。广泛的环加成受体(七个不同类别)参与了化学,化学,区域,非对映和对映选择性生成各种类型的高度有价值的复杂氨基循环。在不分离粗产物的情况下,进行了进一步完善初始氨基循环的多个衍生化反应。最终,我们应用氨基三甲撑甲烷化学方法以8个线性步骤和7.5%的总收率合成了一种潜在的药物,以前是18个线性步骤和0.6%的总收率实现的。
更新日期:2018-07-18
中文翻译:

一种前所未有的氨基-三甲基甲烷化学的去质子化方法:复杂氨基循环的区域,非对映和对映选择性合成
报道了使用脱质子化策略简化氨基-三亚甲基甲烷供体的合成过程,这是从商业和廉价的材料分两个步骤进行的,首次实现了氨基-三甲甲烷化学。广泛的环加成受体(七个不同类别)参与了化学,化学,区域,非对映和对映选择性生成各种类型的高度有价值的复杂氨基循环。在不分离粗产物的情况下,进行了进一步完善初始氨基循环的多个衍生化反应。最终,我们应用氨基三甲撑甲烷化学方法以8个线性步骤和7.5%的总收率合成了一种潜在的药物,以前是18个线性步骤和0.6%的总收率实现的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号