当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst†
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8re00061a V.-L. Yfanti 1, 2, 3, 4, 5 , D. Ipsakis 1, 2, 3, 4, 5 , A. A. Lemonidou 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8re00061a V.-L. Yfanti 1, 2, 3, 4, 5 , D. Ipsakis 1, 2, 3, 4, 5 , A. A. Lemonidou 1, 2, 3, 4, 5
Affiliation
|
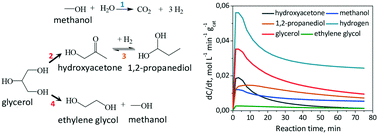
The kinetic modeling of glycerol hydrodeoxygenation reaction over a Cu:Zn:Al catalyst in a batch reactor is reported in this study. The reaction proceeds under inert conditions with hydrogen formed in situ via methanol aqueous phase reforming (APR). A Langmuir–Hinshelwood-type kinetic model, which takes into account the competitive adsorption between reactants and reaction products for the same Cu0 active sites, accurately describes the reaction steps which involve the hydrodeoxygenation of glycerol to 1,2-propanediol via hydroxyacetone as an intermediate, the hydrogenolysis of glycerol to ethylene glycol and the hydrogen formation via the methanol APR reaction. The estimation of the involved kinetic parameters was based on experimental tests performed at different reaction times (0–75 min) and reaction temperatures (473–543 K). The reactions mainly contributing to the formation of 1,2-propanediol were identified as the dehydration of glycerol to hydroxyacetone and its subsequent hydrogenation to the target product. The activation energies for glycerol dehydration and hydroxyacetone hydrogenation reactions were calculated to be 87 and 68.4 kJ mol−1, respectively. Regarding hydrogen formation, the low methanol APR reaction rate was attributed to its low adsorption constant value compared to glycerol and reaction-derived products. The validation of the kinetic model was also investigated by applying it to independent tests with varying glycerol (from 1 to 5 wt%) and methanol (from 7 to 30 wt%) initial concentrations, and the results showed that the model describes the combined reaction cycle for different operating conditions better, when the system is in excess of hydrogen formed via methanol APR.
中文翻译:

铜基催化剂在惰性条件下液相甘油加氢脱氧的动力学研究†
在该研究中报道了在间歇反应器中的Cu:Zn:Al催化剂上甘油加氢脱氧反应的动力学模型。反应在惰性条件下进行,通过甲醇水相重整(APR)原位形成氢。Langmuir-Hinshelwood型动力学模型考虑了相同Cu 0活性位点反应物和反应产物之间的竞争性吸附,准确地描述了反应步骤,其中涉及甘油通过羟基丙酮作为加氢脱氧为1,2-丙二醇。中间体,甘油氢解为乙二醇和氢通过甲醇APR反应。所涉及的动力学参数的估计基于在不同反应时间(0–75分钟)和反应温度(473–543 K)下进行的实验测试。将主要有助于形成1,2-丙二醇的反应鉴定为甘油脱水成羟丙酮,随后氢化成目标产物。甘油脱水和羟丙酮氢化反应的活化能经计算为87和68.4 kJ mol -1, 分别。关于氢的形成,较低的甲醇APR反应速率归因于其与甘油和反应衍生产物相比较低的吸附常数值。还通过将其应用于不同浓度的甘油(1至5 wt%)和甲醇(7至30 wt%)的独立测试中,研究了动力学模型的有效性,结果表明该模型描述了组合反应当系统中通过甲醇APR形成的氢气过量时,对于不同的操作条件循环性更好。
更新日期:2018-06-21
中文翻译:

铜基催化剂在惰性条件下液相甘油加氢脱氧的动力学研究†
在该研究中报道了在间歇反应器中的Cu:Zn:Al催化剂上甘油加氢脱氧反应的动力学模型。反应在惰性条件下进行,通过甲醇水相重整(APR)原位形成氢。Langmuir-Hinshelwood型动力学模型考虑了相同Cu 0活性位点反应物和反应产物之间的竞争性吸附,准确地描述了反应步骤,其中涉及甘油通过羟基丙酮作为加氢脱氧为1,2-丙二醇。中间体,甘油氢解为乙二醇和氢通过甲醇APR反应。所涉及的动力学参数的估计基于在不同反应时间(0–75分钟)和反应温度(473–543 K)下进行的实验测试。将主要有助于形成1,2-丙二醇的反应鉴定为甘油脱水成羟丙酮,随后氢化成目标产物。甘油脱水和羟丙酮氢化反应的活化能经计算为87和68.4 kJ mol -1, 分别。关于氢的形成,较低的甲醇APR反应速率归因于其与甘油和反应衍生产物相比较低的吸附常数值。还通过将其应用于不同浓度的甘油(1至5 wt%)和甲醇(7至30 wt%)的独立测试中,研究了动力学模型的有效性,结果表明该模型描述了组合反应当系统中通过甲醇APR形成的氢气过量时,对于不同的操作条件循环性更好。









































 京公网安备 11010802027423号
京公网安备 11010802027423号