当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A facile 2H-chromene dimerization through an ortho-quinone methide intermediate catalyzed by a sulfonyl derived MIL-101 MOF†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8nj01354c Xin Du 1, 2, 3, 4 , Xiujuan Li 1, 2, 3, 4 , Houliang Tang 5, 6, 7, 8 , Wenyu Wang 8, 9, 10 , Daniele Ramella 8, 11, 12 , Yi Luan 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1039/c8nj01354c Xin Du 1, 2, 3, 4 , Xiujuan Li 1, 2, 3, 4 , Houliang Tang 5, 6, 7, 8 , Wenyu Wang 8, 9, 10 , Daniele Ramella 8, 11, 12 , Yi Luan 1, 2, 3, 4
Affiliation
|
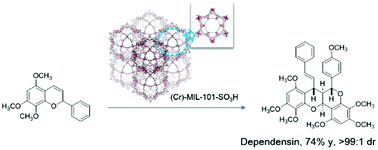
A MIL-101–SO3H MOF was synthesized using commercially available materials. The as-synthesized MIL-101–SO3H was characterized by SEM, XRD, FTIR, BET and TGA. An efficient and diastereoselective homo-dimerization of 2H-chromenes catalysis was achieved using the sulfonyl derived MIL-101 MOF as a catalyst. A benzopyranobenzopyran polycyclic structure was generated in high yield and diastereoselectivity under mild catalytic reaction conditions. Furthermore, a regio-isomer was observed when 2H-chromene bears different substitution groups, furnishing chromeno[2,3-b]chromene dimer in high yield and good diastereoselectivity. A mechanism is proposed with a tandem rearrangement/hetero-Diels–Alder reaction sequence which is supported by evidence. In addition, the acid MIL-101 MOF catalyst can be recycled ten times without compromising the yield and selectivity.
中文翻译:

通过磺酰基衍生的MIL-101 MOF催化的邻甲醌甲基化物中间体进行的 2 H-苯甲基二聚体轻松实现†
MIL-101–SO 3 H MOF是使用市售材料合成的。合成后的MIL-101-SO 3 H的特征在于SEM,XRD,FTIR,BET和TGA。使用磺酰基衍生的MIL-101 MOF作为催化剂,可实现2 H-苯甲基二烯催化的高效非对映选择性均二聚。在温和的催化反应条件下,以高收率和非对映选择性生成了苯并吡喃并苯并吡喃多环结构。此外,当2 H-色烯带有不同的取代基时,观察到一个区域异构体,从而提供了chromeno [2,3- b] chromene二聚体,收率高,非对映选择性好。提出了一种具有串联重排/杂-Diels-Alder反应序列的机制,这得到了证据的支持。此外,酸性MIL-101 MOF催化剂可以循环使用十次,而不会影响收率和选择性。
更新日期:2018-06-21
中文翻译:

通过磺酰基衍生的MIL-101 MOF催化的邻甲醌甲基化物中间体进行的 2 H-苯甲基二聚体轻松实现†
MIL-101–SO 3 H MOF是使用市售材料合成的。合成后的MIL-101-SO 3 H的特征在于SEM,XRD,FTIR,BET和TGA。使用磺酰基衍生的MIL-101 MOF作为催化剂,可实现2 H-苯甲基二烯催化的高效非对映选择性均二聚。在温和的催化反应条件下,以高收率和非对映选择性生成了苯并吡喃并苯并吡喃多环结构。此外,当2 H-色烯带有不同的取代基时,观察到一个区域异构体,从而提供了chromeno [2,3- b] chromene二聚体,收率高,非对映选择性好。提出了一种具有串联重排/杂-Diels-Alder反应序列的机制,这得到了证据的支持。此外,酸性MIL-101 MOF催化剂可以循环使用十次,而不会影响收率和选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号