Food Research International ( IF 7.0 ) Pub Date : 2018-06-21 , DOI: 10.1016/j.foodres.2018.06.044 Yujia Liu , Danyang Ying , Luz Sanguansri , Yanxue Cai , Xueyi Le
|
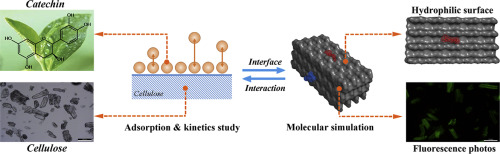
Catechin, an important component of flavan-3-ol, and dietary fiber are both important ingredients with many associated health benefits. The adsorption of catechin onto various dietary fiber has been studied widely, most of the researches focus on the adsorption capacities of catechin under different fibers and the adsorption types by using adsorption models. However, little is known on the dynamic adsorption process and mechanism, including the adsorption sites, interaction types, and participant molecules. In this study, the adsorption behavior and mechanism of catechin onto cellulose were examined by the time function in combination with molecular simulation. The adsorption capacities of cellulose for catechin were 2.70 and 2.82 mg/g at pH 2.0 and 7.0, respectively. The adsorption process was fitted by three stage models (rapid adsorption, saturation, and equilibrium). The features of cellulose and catechin were characterized by FTIR to identify the functional groups in the adsorption. Molecular simulation revealed that the catechin was adsorbed onto the hydrophilic surface of cellulose rather than hydrophobic one, and that the total binding energy was −8.57 kcal/mol of the hydrophilic surface, which was due to Van der Waals' force and H-bond more than electrostatic force. Furthermore, the studies on isothermal adsorption combined with adsorption at various pH illustrated the main interaction between cellulose and catechin for the binding. This work assisted understanding of the adsorption of polyphenols on to insoluble dietary fiber and has the potential of applications in functional foods.
中文翻译:

儿茶素在纤维素上的吸附及其机理研究:动力学模型,表征和分子模拟
儿茶素(黄烷-3-醇的重要成分)和膳食纤维都是重要的成分,具有许多相关的健康益处。儿茶素在各种膳食纤维上的吸附已被广泛研究,大多数研究都通过使用吸附模型来研究儿茶素在不同纤维下的吸附能力和吸附类型。然而,关于动态吸附过程和机理的了解很少,包括吸附位点,相互作用类型和参与分子。本研究通过时间函数结合分子模拟研究了儿茶素在纤维素上的吸附行为和机理。在pH 2.0和7.0时,纤维素对儿茶素的吸附能力分别为2.70和2.82 mg / g。吸附过程由三个阶段的模型拟合(快速吸附,饱和度和平衡)。FTIR表征纤维素和儿茶素的特征,以鉴定吸附中的官能团。分子模拟表明,儿茶素被吸附在纤维素的亲水性表面而不是疏水性表面,并且总的结合能为亲水性表面的-8.57 kcal / mol,这是由于范德华力和氢键所致。比静电力大 此外,等温吸附与在各种pH值下吸附相结合的研究表明,纤维素和儿茶素之间的主要相互作用是结合。这项工作有助于理解多酚在不溶性膳食纤维上的吸附,并具有在功能性食品中应用的潜力。FTIR表征纤维素和儿茶素的特征,以鉴定吸附中的官能团。分子模拟表明,儿茶素被吸附在纤维素的亲水性表面而不是疏水性表面,并且总的结合能为亲水性表面的-8.57 kcal / mol,这是由于范德华力和氢键所致。比静电力大 此外,等温吸附与在各种pH值下吸附相结合的研究表明,纤维素和儿茶素之间的主要相互作用是结合。这项工作有助于理解多酚在不溶性膳食纤维上的吸附,并具有在功能性食品中应用的潜力。FTIR表征纤维素和儿茶素的特征,以鉴定吸附中的官能团。分子模拟表明,儿茶素被吸附在纤维素的亲水性表面而不是疏水性表面,并且总的结合能为亲水性表面的-8.57 kcal / mol,这是由于范德华力和氢键所致。比静电力大 此外,等温吸附与在各种pH值下吸附相结合的研究表明,纤维素和儿茶素之间的主要相互作用是结合。这项工作有助于理解多酚在不溶性膳食纤维上的吸附,并具有在功能性食品中应用的潜力。分子模拟表明,儿茶素被吸附在纤维素的亲水性表面而不是疏水性表面,并且总的结合能为亲水性表面的-8.57 kcal / mol,这是由于范德华力和氢键所致。比静电力大 此外,等温吸附与在各种pH值下吸附结合的研究表明,纤维素和儿茶素之间的主要相互作用是结合。这项工作有助于理解多酚在不溶性膳食纤维上的吸附,并具有在功能性食品中应用的潜力。分子模拟表明,儿茶素被吸附在纤维素的亲水性表面而不是疏水性表面,并且总的结合能为亲水性表面的-8.57 kcal / mol,这是由于范德华力和氢键所致。比静电力大 此外,等温吸附与在各种pH值下吸附相结合的研究表明,纤维素和儿茶素之间的主要相互作用是结合。这项工作有助于理解多酚在不溶性膳食纤维上的吸附,并具有在功能性食品中应用的潜力。此外,等温吸附与在各种pH值下吸附相结合的研究表明,纤维素和儿茶素之间的主要相互作用是结合。这项工作有助于理解多酚在不溶性膳食纤维上的吸附,并具有在功能性食品中应用的潜力。此外,等温吸附与在各种pH值下吸附相结合的研究表明,纤维素和儿茶素之间的主要相互作用是结合。这项工作有助于理解多酚在不溶性膳食纤维上的吸附,并具有在功能性食品中应用的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号