当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the Entropic Effect in Molecular Noncovalent Interactions between Resin-Bound Polybrominated Arenes and Small Substrates.
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-07-09 , DOI: 10.1002/cplu.201800304 Masanori Yamamoto 1, 2, 3 , Miyuki Obara 4 , Keisuke Ochi 2 , Atsushi Yamamoto 4 , Katsuhiko Takenaka 2 , Tsunehiro Tanaka 3 , Kazunori Sato 2
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-07-09 , DOI: 10.1002/cplu.201800304 Masanori Yamamoto 1, 2, 3 , Miyuki Obara 4 , Keisuke Ochi 2 , Atsushi Yamamoto 4 , Katsuhiko Takenaka 2 , Tsunehiro Tanaka 3 , Kazunori Sato 2
Affiliation
|
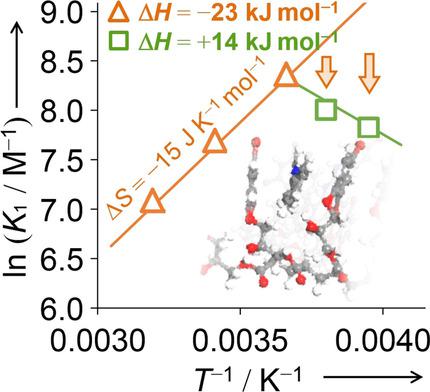
The associative interaction between resin-bound polybrominated arenes and small molecules was analyzed by using various spectroscopic techniques as well as a synthetic molecular model to establish the thermodynamics. The binding in acetonitrile was three orders of magnitude stronger than that in methanol, partly owing to the tertiary conformational gating of the resin that controls the entropic terms. By using the entropic superiority, the associative binding of up to 3×104 m-1 is achieved with the non-biological system. A modified Hill plot for the quantitative analysis of bindings was also devised, which enabled the interactions at the molecular level to be elucidated.
中文翻译:

探究树脂结合的多溴芳烃与小底物之间分子非共价相互作用中的熵效应。
树脂结合的多溴代芳烃和小分子之间的缔合相互作用通过使用各种光谱技术以及建立热力学的合成分子模型进行了分析。乙腈中的结合力比甲醇中的结合力强三个数量级,部分是由于控制熵项的树脂的三级构象门控。通过利用熵的优势,利用非生物系统可以实现高达3×104 m-1的关联结合。还设计了修改后的希尔图,用于结合的定量分析,这使得在分子水平上的相互作用得以阐明。
更新日期:2018-07-09
中文翻译:

探究树脂结合的多溴芳烃与小底物之间分子非共价相互作用中的熵效应。
树脂结合的多溴代芳烃和小分子之间的缔合相互作用通过使用各种光谱技术以及建立热力学的合成分子模型进行了分析。乙腈中的结合力比甲醇中的结合力强三个数量级,部分是由于控制熵项的树脂的三级构象门控。通过利用熵的优势,利用非生物系统可以实现高达3×104 m-1的关联结合。还设计了修改后的希尔图,用于结合的定量分析,这使得在分子水平上的相互作用得以阐明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号