当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of a 2,3-bis(pyridyl)pyrazinyl chelate bridging ligand in the reactivity of Ru(ii)–Pt(ii) dinuclear complexes on the substitution of chlorides by thiourea nucleophiles – a kinetic study†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1039/c8nj02096e Rajesh Bellam 1, 2, 3, 4 , Deogratius Jaganyi 5, 6, 7, 8 , Allen Mambanda 1, 2, 3, 4 , Ross Robinson 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1039/c8nj02096e Rajesh Bellam 1, 2, 3, 4 , Deogratius Jaganyi 5, 6, 7, 8 , Allen Mambanda 1, 2, 3, 4 , Ross Robinson 1, 2, 3, 4
Affiliation
|
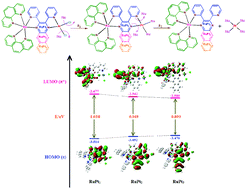
Chloride substitution from [(1,10-phenanthroline)2Ru(II)(μ-2,3-bis(2-pyridyl)pyrazine)Pt(II)dichloride]2+ (RuPt1), [(1,10-phenanthroline)2Ru(II)(μ-2,3-bis(2-pyridyl)quinoxaline)Pt(II)dichloride]2+ (RuPt2) and [(1,10-phenanthroline)2Ru(II)(μ-2,3-bis(2-pyridyl)benzo[g]quinoxaline)Pt(II)dichloride]2+ (RuPt3) by thiourea (TU), 1,3-dimethyl-2-thioura (DMTU) and 1,1,3,3-tetra methyl-2-thiourea (TMTU) was studied in a methanol medium (I = 0.10 M) under pseudo-first-order conditions. The rate of substitution was investigated as a function of concentration of nucleophile and temperature using the stopped-flow technique. Two consecutive substitution steps were observed. The first and fastest step was ascribed to the simultaneous substitution of the two chloride co-ligands by incoming nucleophiles according to the rate law: k1stobs = k1st2[Nu]. The subsequent step was assigned to the dechelation of the rigid 2,3-bis(pyridyl)pyrazinyl bridging ligand from the Pt(II) centres of the substituted intermediates to give Pt(Nu)42+ and (phen)2Ru(II)(2,3-bis(pyridyl)pyrazinyl) groups as products. The rate law for this step is k2ndobs = k2nd2[Nu] + k2nd−2. The second-order kinetics and large negative entropies for both steps support an associative mechanism of substitution. The rate of chloride substitution was RuPt1 ≪ RuPt2 < RuPt3. This is also the order of increase in the π-surface of the bridging ligand and an indication that π-back donation of electron density from the Pt-5dπ orbitals into the π*-acceptor bridging ligand is the dominant factor controlling the substitution of the chloride from the complexes. The nucleophiles’ order of reactivity was TU > DMTU > TMTU, in accordance with their steric bulk.
中文翻译:

2,3-双(吡啶基)吡嗪基螯合物桥联配体在Ru(ii)-Pt(ii)双核络合物对硫脲亲核试剂取代氯化物的反应性中的作用–动力学研究†
[[(1,10-菲咯啉)2 Ru(II)(μ-2,3-双(2-吡啶基)吡嗪)Pt(II)二氯化物] 2+(RuPt 1),[(1,10-菲咯啉)2 Ru(II)(μ-2,3-双(2-吡啶基)喹喔啉)Pt(II)二氯化物] 2+(RuPt 2)和[(1,10-菲咯啉)2 Ru(II)(μ -2,3-双(2-吡啶基)苯并[ g ]喹喔啉)Pt(II)二氯化物] 2+(RuPt 3)由硫脲(TU),1,3-二甲基-2-硫脲(DMTU)和1,1,3,3-四甲基-2-硫脲(TMTU)在甲醇介质(I = 0.10 M)下于伪一阶条件。使用停止流技术研究了取代率与亲核试剂浓度和温度的关系。观察到两个连续的取代步骤。第一步和最快的步骤是根据速率定律:传入亲核试剂同时取代两个氯化物共配体:k 1st obs = k 1st 2 [Nu]。后续步骤分配给Pt(II)的刚性2,3-双(吡啶基)吡嗪基桥联配体的螯合)取代中间体的中心,得到Pt(Nu)4 2+和(phen)2 Ru(II)(2,3-双(吡啶基)吡嗪基)基团。该步骤的速率定律是k 2nd obs = k 2nd 2 [Nu] + k 2nd -2。这两个步骤的二阶动力学和大的负熵都支持取代的关联机制。氯取代率为RUPT 1 « RUPT 2 < RUPT 3。这也是桥联配体的π表面增加的顺序,并且表明从Pt-5dπ轨道向π*受体桥联配体的π向电子回输是控制该键的取代的主要因素。络合物中的氯化物。亲核试剂的反应顺序为TU> DMTU> TMTU,这取决于它们的空间体积。
更新日期:2018-06-20
中文翻译:

2,3-双(吡啶基)吡嗪基螯合物桥联配体在Ru(ii)-Pt(ii)双核络合物对硫脲亲核试剂取代氯化物的反应性中的作用–动力学研究†
[[(1,10-菲咯啉)2 Ru(II)(μ-2,3-双(2-吡啶基)吡嗪)Pt(II)二氯化物] 2+(RuPt 1),[(1,10-菲咯啉)2 Ru(II)(μ-2,3-双(2-吡啶基)喹喔啉)Pt(II)二氯化物] 2+(RuPt 2)和[(1,10-菲咯啉)2 Ru(II)(μ -2,3-双(2-吡啶基)苯并[ g ]喹喔啉)Pt(II)二氯化物] 2+(RuPt 3)由硫脲(TU),1,3-二甲基-2-硫脲(DMTU)和1,1,3,3-四甲基-2-硫脲(TMTU)在甲醇介质(I = 0.10 M)下于伪一阶条件。使用停止流技术研究了取代率与亲核试剂浓度和温度的关系。观察到两个连续的取代步骤。第一步和最快的步骤是根据速率定律:传入亲核试剂同时取代两个氯化物共配体:k 1st obs = k 1st 2 [Nu]。后续步骤分配给Pt(II)的刚性2,3-双(吡啶基)吡嗪基桥联配体的螯合)取代中间体的中心,得到Pt(Nu)4 2+和(phen)2 Ru(II)(2,3-双(吡啶基)吡嗪基)基团。该步骤的速率定律是k 2nd obs = k 2nd 2 [Nu] + k 2nd -2。这两个步骤的二阶动力学和大的负熵都支持取代的关联机制。氯取代率为RUPT 1 « RUPT 2 < RUPT 3。这也是桥联配体的π表面增加的顺序,并且表明从Pt-5dπ轨道向π*受体桥联配体的π向电子回输是控制该键的取代的主要因素。络合物中的氯化物。亲核试剂的反应顺序为TU> DMTU> TMTU,这取决于它们的空间体积。











































 京公网安备 11010802027423号
京公网安备 11010802027423号