当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Modification of Dehydroalanine in Peptides and Proteins by Palladium‐Mediated Cross‐Coupling
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-07-18 , DOI: 10.1002/chem.201802846 A. Dowine de Bruijn 1 , Gerard Roelfes 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-07-18 , DOI: 10.1002/chem.201802846 A. Dowine de Bruijn 1 , Gerard Roelfes 1
Affiliation
|
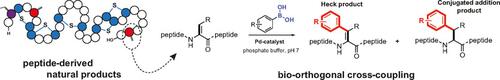
Dehydroalanine (Dha) is a remarkably versatile non‐canonical amino acid often found in antimicrobial peptides. Herein, we present the catalytic modification of Dha by a palladium‐mediated cross‐coupling reaction. By using Pd(EDTA)(OAc)2 as water‐soluble catalyst, a variety of arylboronic acids was coupled to the dehydrated residues in proteins and peptides, such as Nisin. The cross‐coupling reaction gave both the Heck product, in which the sp2‐hybridisation of the α‐carbon is retained, as well as the conjugated addition product. The reaction can be performed under mild aqueous conditions, which makes this method an attractive addition to the palette of bio‐orthogonal catalytic methods.
中文翻译:

钯介导的交叉偶联催化多肽和蛋白质中脱氢丙氨酸的催化修饰
脱氢丙氨酸(Dha)是一种非常通用的非规范氨基酸,通常在抗菌肽中发现。本文中,我们介绍了通过钯介导的交叉偶联反应对Dha的催化修饰。通过使用Pd(EDTA)(OAc)2作为水溶性催化剂,多种芳基硼酸与蛋白质和多肽(如乳链菌肽)中的脱水残基偶联。交叉偶联反应既产生了保留了α-碳的sp 2-杂化的Heck产物,也产生了共轭加成产物。该反应可在温和的水性条件下进行,这使该方法成为生物正交催化方法的一个有吸引力的补充。
更新日期:2018-07-18
中文翻译:

钯介导的交叉偶联催化多肽和蛋白质中脱氢丙氨酸的催化修饰
脱氢丙氨酸(Dha)是一种非常通用的非规范氨基酸,通常在抗菌肽中发现。本文中,我们介绍了通过钯介导的交叉偶联反应对Dha的催化修饰。通过使用Pd(EDTA)(OAc)2作为水溶性催化剂,多种芳基硼酸与蛋白质和多肽(如乳链菌肽)中的脱水残基偶联。交叉偶联反应既产生了保留了α-碳的sp 2-杂化的Heck产物,也产生了共轭加成产物。该反应可在温和的水性条件下进行,这使该方法成为生物正交催化方法的一个有吸引力的补充。











































 京公网安备 11010802027423号
京公网安备 11010802027423号