当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of substituted tetrahydro-1-benzazepines by lithiation-trapping
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-08-17 , DOI: 10.1002/ejoc.201800868 Tahani Aeyad 1 , Callum G. Jones 1 , Iain Coldham 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-08-17 , DOI: 10.1002/ejoc.201800868 Tahani Aeyad 1 , Callum G. Jones 1 , Iain Coldham 1
Affiliation
|
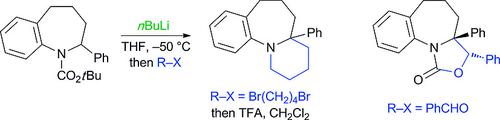
The tetrahydro‐1‐benzazepine or benzo[b]azepine ring system is found in a number of drug molecules although methods to access 2,2‐disubstituted derivatives are rare. Here we report the preparation of N‐tert‐butoxycarbonyl‐2‐phenyltetrahydro‐1‐benzazepine followed by lithiation and trapping with electrophiles. The metallation reaction was optimized by using React‐IR spectroscopy, and VT‐NMR spectroscopy allowed the determination of the rate of rotation of the Boc group (approximate ΔG‡ 63 kJ/mol at –50 °C). The resulting organolithium was quenched to give either 2,2‐disubstituted products or, with certain electrophiles, the ortho‐substituted products, presumably through an η3‐coordinated benzyllithium intermediate. The chemistry was shown to be amenable to extension to the 7‐methoxy analog. Removal of the Boc group from the nitrogen atom led to amine products.
中文翻译:

锂化捕获法制备取代的四氢-1-苯并氮杂
四氢-1-苯并氮杂或苯并[b]氮杂环系统存在于许多药物分子中,尽管获得2,2-二取代衍生物的方法很少见。在这里,我们报告了 N-叔丁氧基羰基-2-苯基四氢-1-苯并氮杂的制备,然后锂化并用亲电试剂捕获。通过使用 React-IR 光谱优化金属化反应,并且 VT-NMR 光谱允许确定 Boc 基团的旋转速率(在 –50 °C 时约为 ΔG‡ 63 kJ/mol)。将所得的有机锂猝灭以得到 2,2-二取代产物,或者使用某些亲电子试剂得到邻位取代产物,可能是通过 η3 配位的苄基锂中间体。表明该化学反应适合扩展到 7-甲氧基类似物。
更新日期:2018-08-17
中文翻译:

锂化捕获法制备取代的四氢-1-苯并氮杂
四氢-1-苯并氮杂或苯并[b]氮杂环系统存在于许多药物分子中,尽管获得2,2-二取代衍生物的方法很少见。在这里,我们报告了 N-叔丁氧基羰基-2-苯基四氢-1-苯并氮杂的制备,然后锂化并用亲电试剂捕获。通过使用 React-IR 光谱优化金属化反应,并且 VT-NMR 光谱允许确定 Boc 基团的旋转速率(在 –50 °C 时约为 ΔG‡ 63 kJ/mol)。将所得的有机锂猝灭以得到 2,2-二取代产物,或者使用某些亲电子试剂得到邻位取代产物,可能是通过 η3 配位的苄基锂中间体。表明该化学反应适合扩展到 7-甲氧基类似物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号