当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomistic Modeling of F-Actin Mechanical Responses and Determination of Mechanical Properties
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00640 Si Li 1 , Jin Zhang 2 , Chengyuan Wang 1 , Perumal Nithiarasu 1
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acsbiomaterials.8b00640 Si Li 1 , Jin Zhang 2 , Chengyuan Wang 1 , Perumal Nithiarasu 1
Affiliation
|
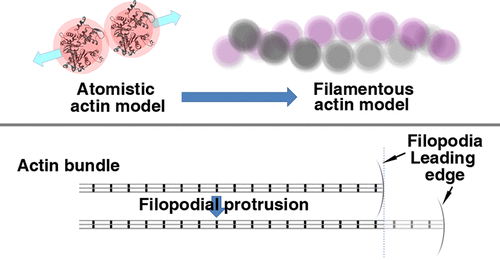
A molecular structural mechanics (MSM) model was developed for F-actins in cells, where the force constants describing the monomer interaction were achieved using molecular dynamics simulations. The MSM was then employed to predict the mechanical properties of F-actin. The obtained Young’s modulus (1.92 GPa), torsional rigidity (2.36 × 10–26 Nm2), and flexural rigidity (10.84 × 10–26 Nm2) were found to be in good agreement with existing experimental data. Subsequently, the tension-induced bending was studied for F-actins as a result of their helical structure. Mechanical instability was also investigated for the actin filaments in filopodial protrusion by considering the reinforcing effect of the actin-binding proteins. The predicted buckling load agreed well with the experimentally obtained stall force, showing a pivotal role of the actin-binding protein in regulating the stiffness of F-actin bundles during the formation of filopodia protrusion. Herein, it is expected that the MSM model can be extended to the mechanics of more complex filamentous systems such as stress fibers and actin meshwork.
中文翻译:

F-Actin力学响应的原子建模及力学性能的确定
针对细胞中的F-肌动蛋白开发了分子结构力学(MSM)模型,其中使用分子动力学模拟获得了描述单体相互作用的力常数。然后使用MSM预测F-肌动蛋白的机械性能。获得的杨氏模量(1.92 GPa),抗扭刚度(2.36×10 –26 Nm 2)和抗弯刚度(10.84×10 –26 Nm 2))与现有实验数据非常吻合。随后,研究了由于F-肌动蛋白的螺旋结构而导致的张力诱导的弯曲。通过考虑肌动蛋白结合蛋白的增强作用,还研究了腓肠肌前突肌动蛋白丝的机械不稳定性。预测的屈曲载荷与实验获得的失速力很好地吻合,表明肌动蛋白结合蛋白在丝状伪足突起形成过程中在调节F-肌动蛋白束的硬度中起关键作用。在此,期望MSM模型可以扩展到更复杂的丝状系统的力学,例如应力纤维和肌动蛋白网。
更新日期:2018-06-19
中文翻译:

F-Actin力学响应的原子建模及力学性能的确定
针对细胞中的F-肌动蛋白开发了分子结构力学(MSM)模型,其中使用分子动力学模拟获得了描述单体相互作用的力常数。然后使用MSM预测F-肌动蛋白的机械性能。获得的杨氏模量(1.92 GPa),抗扭刚度(2.36×10 –26 Nm 2)和抗弯刚度(10.84×10 –26 Nm 2))与现有实验数据非常吻合。随后,研究了由于F-肌动蛋白的螺旋结构而导致的张力诱导的弯曲。通过考虑肌动蛋白结合蛋白的增强作用,还研究了腓肠肌前突肌动蛋白丝的机械不稳定性。预测的屈曲载荷与实验获得的失速力很好地吻合,表明肌动蛋白结合蛋白在丝状伪足突起形成过程中在调节F-肌动蛋白束的硬度中起关键作用。在此,期望MSM模型可以扩展到更复杂的丝状系统的力学,例如应力纤维和肌动蛋白网。











































 京公网安备 11010802027423号
京公网安备 11010802027423号