当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competition between H and CO for Active Sites Governs Copper‐Mediated Electrosynthesis of Hydrocarbon Fuels
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201806051 Marcel Schreier 1 , Youngmin Yoon 1 , Megan N. Jackson 1 , Yogesh Surendranath 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201806051 Marcel Schreier 1 , Youngmin Yoon 1 , Megan N. Jackson 1 , Yogesh Surendranath 1
Affiliation
|
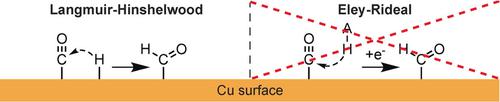
The dynamics of carbon monoxide on Cu surfaces was investigated during CO reduction, providing insight into the mechanism leading to the formation of hydrogen, methane, and ethylene, the three key products in the electrochemical reduction of CO2. Reaction order experiments were conducted at low temperature in an ethanol medium affording high solubility and surface‐affinity for carbon monoxide. Surprisingly, the methane production rate is suppressed by increasing the pressure of CO, whereas ethylene production remains largely unaffected. The data show that CH4 and H2 production are linked through a common H intermediate and that methane is formed through reactions among adsorbed H and CO, which are in direct competition with each other for surface sites. The data exclude the participation of solution species in rate‐limiting steps, highlighting the importance of increasing surface recombination rates for efficient fuel synthesis.
中文翻译:

氢和一氧化碳在活性位点之间的竞争决定了铜介导的碳氢化合物燃料的电合成
在CO还原过程中研究了一氧化碳在Cu表面的动力学,从而深入了解了导致氢,甲烷和乙烯形成的机理,这是电化学还原CO 2的三个关键产物。反应顺序实验在低温下在提供一氧化碳高溶解度和表面亲和力的乙醇介质中进行。出人意料的是,通过增加CO的压力抑制了甲烷的产生速率,而乙烯的产生基本上未受影响。数据显示CH 4和H 2生产过程通过共同的H中间体连接,甲烷是通过吸附的H和CO之间的反应形成的,而H和CO在表面位点上彼此直接竞争。数据不包括溶液种类参与限速步骤,突显了提高表面重组率对有效燃料合成的重要性。
更新日期:2018-07-13
中文翻译:

氢和一氧化碳在活性位点之间的竞争决定了铜介导的碳氢化合物燃料的电合成
在CO还原过程中研究了一氧化碳在Cu表面的动力学,从而深入了解了导致氢,甲烷和乙烯形成的机理,这是电化学还原CO 2的三个关键产物。反应顺序实验在低温下在提供一氧化碳高溶解度和表面亲和力的乙醇介质中进行。出人意料的是,通过增加CO的压力抑制了甲烷的产生速率,而乙烯的产生基本上未受影响。数据显示CH 4和H 2生产过程通过共同的H中间体连接,甲烷是通过吸附的H和CO之间的反应形成的,而H和CO在表面位点上彼此直接竞争。数据不包括溶液种类参与限速步骤,突显了提高表面重组率对有效燃料合成的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号