当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiourea‐Catalyzed Asymmetric Michael Addition of Carbazolones to 2‐Chloroacrylonitrile: Total Synthesis of 5,22‐Dioxokopsane, Kopsinidine C, and Demethoxycarbonylkopsin
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-11 , DOI: 10.1002/anie.201805905 Dongshun Ni 1 , Yi Wei 1 , Dawei Ma 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-11 , DOI: 10.1002/anie.201805905 Dongshun Ni 1 , Yi Wei 1 , Dawei Ma 1
Affiliation
|
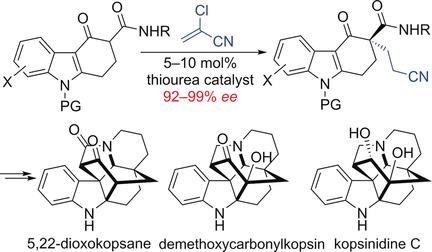
A modified Takemoto catalyst enabled the asymmetric Michael addition of carbazolones to 2‐chloroacrylonitrile to afford 3,3‐disubstituted carbazolones with excellent enantioselectivity. This method was successfully applied to total syntheses of three Kopsia alkaloids which featured an unprecedented MnIII‐mediated oxidative cyclization to create the caged ring system and a SmI2‐mediated reductive coupling as key steps.
中文翻译:

硫脲催化的咔唑酮向2-氯丙烯腈的不对称迈克尔加成反应:5,22-二氧代正庚烷,甲庚啶C和脱甲氧基羰基甲酚的全合成
改性的Takemoto催化剂能够使咔唑酮不对称地迈克尔加成到2-氯丙烯腈中,从而提供具有出色对映选择性的3,3-二取代咔唑酮。此方法已成功应用于三种Kopsia生物碱的全合成,这些生物碱具有史无前例的Mn III介导的氧化环化作用,以形成笼状环系统和SmI 2介导的还原偶联为关键步骤。
更新日期:2018-07-11
中文翻译:

硫脲催化的咔唑酮向2-氯丙烯腈的不对称迈克尔加成反应:5,22-二氧代正庚烷,甲庚啶C和脱甲氧基羰基甲酚的全合成
改性的Takemoto催化剂能够使咔唑酮不对称地迈克尔加成到2-氯丙烯腈中,从而提供具有出色对映选择性的3,3-二取代咔唑酮。此方法已成功应用于三种Kopsia生物碱的全合成,这些生物碱具有史无前例的Mn III介导的氧化环化作用,以形成笼状环系统和SmI 2介导的还原偶联为关键步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号