当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into the Nature of Oxidant and Mechanism in the Regioselective Syn‐dihydroxylation of an Alkene with a Rieske oxygenase inspired Iron Catalyst
ChemCatChem ( IF 3.8 ) Pub Date : 2018-07-10 , DOI: 10.1002/cctc.201800799 Lisa Roy 1
ChemCatChem ( IF 3.8 ) Pub Date : 2018-07-10 , DOI: 10.1002/cctc.201800799 Lisa Roy 1
Affiliation
|
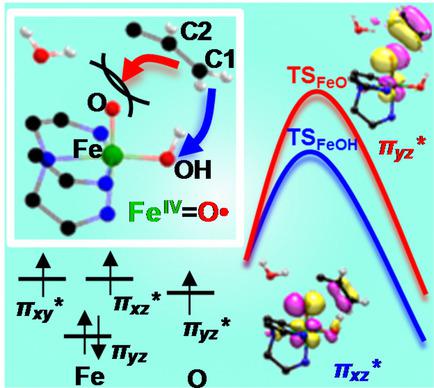
Selective C=C bond oxidation is a fundamental process in Nature and has potential utility in organic synthesis. Herein, ab initio and density functional techniques are employed to investigate the nature of the oxidant and mechanism in alkene oxidation by two H2O2 activating Rieske oxygenase inspired iron catalysts, one of which is more sterically encumbered. Electronic structure analysis reveals that the mechanism involves a water‐assisted homolytic O−O bond cleavage of the peroxide moiety to generate an FeIV(O.)OH radical intermediate, followed by epoxidation or syn‐dihydroxylation of the alkene substrate. Furthermore, it is shown that a greater degree of structural reorganization due to higher steric repulsion between the substrate and bulky substituents selectively avoids epoxidation in one of the complexes, thus directing it towards exclusive hydroxylation. Additionally, the rate‐determining barrier for cis‐diol release is curtailed by ∼14 kcal/mol due to synergistic assistance from Lewis acid. Our study therefore highlights the crucial role of ligand modification and additives which can be further engineered to achieve enzyme‐like optimum activity.
中文翻译:

对具有Rieske加氧酶启发的铁催化剂的烯烃区域选择性合成二羟基化反应的氧化剂性质和机理的理论见解
选择性的C = C键氧化是自然界中的基本过程,在有机合成中具有潜在的实用性。本文中,从头算和密度泛函技术用于研究氧化剂的性质和通过两种H 2 O 2活化Rieske加氧酶激发的铁催化剂在烯烃氧化中的机理,其中一种在空间上较为繁琐。电子结构分析表明,该机制涉及过氧化物部分以生成Fe的水辅助均裂O-O键裂解IV(O 。)OH基团的中间体,然后再环氧化或顺烯烃底物的二羟基化。此外,显示出由于底物和庞大的取代基之间较高的空间排斥而导致的更大程度的结构重组选择性地避免了其中一种配合物中的环氧化,从而将其引导至排他性羟基化。此外,由于路易斯酸的协同协助,决定顺式二醇释放的速率障碍降低了约14 kcal / mol。因此,我们的研究强调了配体修饰和添加剂的关键作用,可以对其进行进一步改造以实现类似酶的最佳活性。
更新日期:2018-07-10
中文翻译:

对具有Rieske加氧酶启发的铁催化剂的烯烃区域选择性合成二羟基化反应的氧化剂性质和机理的理论见解
选择性的C = C键氧化是自然界中的基本过程,在有机合成中具有潜在的实用性。本文中,从头算和密度泛函技术用于研究氧化剂的性质和通过两种H 2 O 2活化Rieske加氧酶激发的铁催化剂在烯烃氧化中的机理,其中一种在空间上较为繁琐。电子结构分析表明,该机制涉及过氧化物部分以生成Fe的水辅助均裂O-O键裂解IV(O 。)OH基团的中间体,然后再环氧化或顺烯烃底物的二羟基化。此外,显示出由于底物和庞大的取代基之间较高的空间排斥而导致的更大程度的结构重组选择性地避免了其中一种配合物中的环氧化,从而将其引导至排他性羟基化。此外,由于路易斯酸的协同协助,决定顺式二醇释放的速率障碍降低了约14 kcal / mol。因此,我们的研究强调了配体修饰和添加剂的关键作用,可以对其进行进一步改造以实现类似酶的最佳活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号