当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of Pyrophosphate‐Targeting Vancomycin Derivatives for Combating Vancomycin‐Resistant Enterococci
ChemMedChem ( IF 3.6 ) Pub Date : 2018-07-17 , DOI: 10.1002/cmdc.201800252 Dongliang Guan 1, 2 , Feifei Chen 1 , Faridoon 1, 2 , Junjie Liu 1, 3 , Jian Li 1, 2 , Lefu Lan 1, 2, 4 , Wei Huang 1, 2, 5
ChemMedChem ( IF 3.6 ) Pub Date : 2018-07-17 , DOI: 10.1002/cmdc.201800252 Dongliang Guan 1, 2 , Feifei Chen 1 , Faridoon 1, 2 , Junjie Liu 1, 3 , Jian Li 1, 2 , Lefu Lan 1, 2, 4 , Wei Huang 1, 2, 5
Affiliation
|
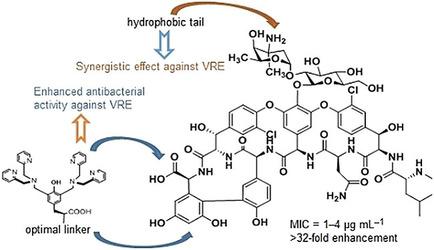
As the last resort for intractable Gram‐positive bacterial infections, vancomycin is losing efficacy with the emergence of vancomycin‐resistant bacteria, especially vancomycin‐resistant Enterococci (VRE). To combat this threat, we rationally designed and synthesized 39 novel vancomycin derivatives by respective or combined modifications using metal‐chelating, lipophilic, and galactose‐attachment strategies for extensive structure–activity relationship (SAR) analysis. In a proposed mechanism, the conjugation of dipicolylamine on the seventh amino acid resorcinol position or C‐terminus endowed the vancomycin backbone with binding capacity for the pyrophosphate moiety in lipid II while maintaining the intrinsic binding affinity for the dipeptide terminus of the bacterial cell wall peptidoglycan precursor. The in vitro antibacterial activities were evaluated, and the optimal compounds indicated 16‐ to 1024‐fold higher activity against VRE than that of vancomycin. Compound 11 b (3′,5′‐bis(dipicolylaminomethyl)tyrosine [1,2,3]triazolylmethoxylethyoxyl ethylaminomethyl‐N‐decylvancomycin) was found to have particularly potent activity against VRE through synergistic effects brought about by combining two peripheral modifications.
中文翻译:

靶向抗焦磷酸的万古霉素衍生物的设计与合成
作为难治性革兰氏阳性细菌感染的最后手段,随着耐万古霉素细菌的出现,特别是耐万古霉素肠球菌的出现,万古霉素正在失去疗效(VRE)。为了克服这种威胁,我们通过金属螯合,亲脂性和半乳糖附着策略,分别或联合修饰,合理设计和合成了39种新的万古霉素衍生物,以进行广泛的结构-活性关系(SAR)分析。在一个拟议的机制中,二聚烯丙基胺在间苯二酚的第七个氨基酸位置或C-末端上的缀合使万古霉素骨架具有对脂质II中焦磷酸部分的结合能力,同时保持了对细菌细胞壁肽聚糖二肽末端的固有结合亲和力前体。对体外抗菌活性进行了评估,最佳化合物显示对VRE的活性比万古霉素高16到1024倍。化合物11 b(3',5'-双(二吡啶甲酰基氨基甲基)酪氨酸[1,2,3]三唑基甲氧基乙氧基乙基氨基甲基-N-癸基万古霉素)通过结合两个外围修饰而产生的协同作用,对VRE具有特别强的活性。
更新日期:2018-07-17
中文翻译:

靶向抗焦磷酸的万古霉素衍生物的设计与合成
作为难治性革兰氏阳性细菌感染的最后手段,随着耐万古霉素细菌的出现,特别是耐万古霉素肠球菌的出现,万古霉素正在失去疗效(VRE)。为了克服这种威胁,我们通过金属螯合,亲脂性和半乳糖附着策略,分别或联合修饰,合理设计和合成了39种新的万古霉素衍生物,以进行广泛的结构-活性关系(SAR)分析。在一个拟议的机制中,二聚烯丙基胺在间苯二酚的第七个氨基酸位置或C-末端上的缀合使万古霉素骨架具有对脂质II中焦磷酸部分的结合能力,同时保持了对细菌细胞壁肽聚糖二肽末端的固有结合亲和力前体。对体外抗菌活性进行了评估,最佳化合物显示对VRE的活性比万古霉素高16到1024倍。化合物11 b(3',5'-双(二吡啶甲酰基氨基甲基)酪氨酸[1,2,3]三唑基甲氧基乙氧基乙基氨基甲基-N-癸基万古霉素)通过结合两个外围修饰而产生的协同作用,对VRE具有特别强的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号