当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Increasing Enzyme Stability and Activity through Hydrogen Bond-Enhanced Halogen Bonds.
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-03 , DOI: 10.1021/acs.biochem.8b00603 Anna-Carin C Carlsson 1 , Matthew R Scholfield 1 , Rhianon K Rowe 1 , Melissa Coates Ford 1 , Austin T Alexander 2 , Ryan A Mehl 2 , P Shing Ho 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-03 , DOI: 10.1021/acs.biochem.8b00603 Anna-Carin C Carlsson 1 , Matthew R Scholfield 1 , Rhianon K Rowe 1 , Melissa Coates Ford 1 , Austin T Alexander 2 , Ryan A Mehl 2 , P Shing Ho 1
Affiliation
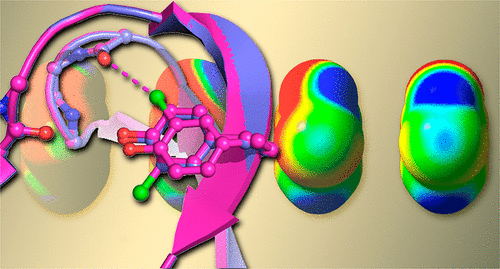
|
The construction of more stable proteins is important in biomolecular engineering, particularly in the design of biologics-based therapeutics. We show here that replacing the tyrosine at position 18 (Y18) of T4 lysozyme with the unnatural amino acid m-chlorotyrosine ( mClY) increases both the thermal stability (increasing the melting temperature by ∼1 °C and the melting enthalpy by 3 kcal/mol) and the enzymatic activity at elevated temperatures (15% higher than that of the parent enzyme at 40 °C) of this classic enzyme. The chlorine of mClY forms a halogen bond (XB) to the carbonyl oxygen of the peptide bond at glycine 28 (G28) in a tight loop near the active site. In this case, the XB potential of the typically weak XB donor Cl is shown from quantum chemical calculations to be significantly enhanced by polarization via an intramolecular hydrogen bond (HB) from the adjacent hydroxyl substituent of the tyrosyl side chain, resulting in a distinctive synergistic HB-enhanced XB (or HeX-B for short) interaction. The larger halogens (bromine and iodine) are not well accommodated within this same loop and, consequently, do not exhibit the effects on protein stability or function associated with the HeX-B interaction. Thus, we have for the first time demonstrated that an XB can be engineered to stabilize and increase the activity of an enzyme, with the increased stabilizing potential of the HeX-B further extending the application of halogenated amino acids in the design of more stable protein therapeutics.
中文翻译:

通过氢键增强的卤素键提高酶的稳定性和活性。
在生物分子工程中,尤其是在基于生物制剂的治疗剂的设计中,更稳定的蛋白质的构建非常重要。我们在这里显示,用非天然氨基酸间氯酪氨酸(mClY)代替T4溶菌酶18位(Y18)的酪氨酸既提高了热稳定性(将熔化温度提高了约1°C,又将熔化焓提高了3 kcal /摩尔比)和这种经典酶在高温下(40℃下比亲本酶高15%)的酶活性。mClY的氯在活性位点附近的紧密环中与甘氨酸28(G28)上肽键的羰基氧形成卤素键(XB)。在这种情况下,从量子化学计算中可以看出,通常较弱的XB供体C1的XB势能通过来自酪氨酰侧链相邻羟基取代基的分子内氢键(HB)极化而显着增强,从而产生了独特的HB协同增效XB (或简称为HeX-B)交互。较大的卤素(溴和碘)不能很好地容纳在同一环中,因此不会对蛋白质稳定性或与HeX-B相互作用相关的功能产生影响。因此,我们首次证明XB可以被工程化以稳定和增加酶的活性,而HeX-B的增加的稳定潜力进一步扩大了卤代氨基酸在更稳定的蛋白质设计中的应用。疗法。
更新日期:2018-06-19
中文翻译:

通过氢键增强的卤素键提高酶的稳定性和活性。
在生物分子工程中,尤其是在基于生物制剂的治疗剂的设计中,更稳定的蛋白质的构建非常重要。我们在这里显示,用非天然氨基酸间氯酪氨酸(mClY)代替T4溶菌酶18位(Y18)的酪氨酸既提高了热稳定性(将熔化温度提高了约1°C,又将熔化焓提高了3 kcal /摩尔比)和这种经典酶在高温下(40℃下比亲本酶高15%)的酶活性。mClY的氯在活性位点附近的紧密环中与甘氨酸28(G28)上肽键的羰基氧形成卤素键(XB)。在这种情况下,从量子化学计算中可以看出,通常较弱的XB供体C1的XB势能通过来自酪氨酰侧链相邻羟基取代基的分子内氢键(HB)极化而显着增强,从而产生了独特的HB协同增效XB (或简称为HeX-B)交互。较大的卤素(溴和碘)不能很好地容纳在同一环中,因此不会对蛋白质稳定性或与HeX-B相互作用相关的功能产生影响。因此,我们首次证明XB可以被工程化以稳定和增加酶的活性,而HeX-B的增加的稳定潜力进一步扩大了卤代氨基酸在更稳定的蛋白质设计中的应用。疗法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号