当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of Potent Anti-HER1/2 Immunotoxins by Protein Ligation Using Split Inteins
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acschembio.8b00222 Thomas Pirzer 1 , Kira-Sophie Becher 2 , Marcel Rieker 3, 4 , Tobias Meckel 5 , Henning D Mootz 2 , Harald Kolmar 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acschembio.8b00222 Thomas Pirzer 1 , Kira-Sophie Becher 2 , Marcel Rieker 3, 4 , Tobias Meckel 5 , Henning D Mootz 2 , Harald Kolmar 1
Affiliation
|
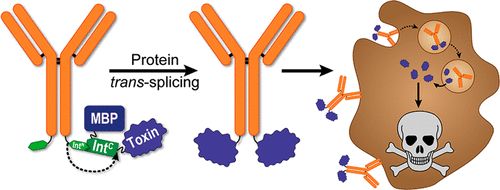
Cell targeting protein toxins have gained increasing interest for cancer therapy aimed at increasing the therapeutic window and reducing systemic toxicity. Because recombinant expression of immunotoxins consisting of a receptor-binding and a cell-killing moiety is hampered by their high toxicity in a eukaryotic production host, most applications rely on recombinant production of fusion proteins consisting of an antibody fragment and a protein toxin in bacterial hosts such as Escherichia coli (E. coli). These fusions often lack beneficial properties of whole antibodies like extended serum half-life or efficient endocytic uptake via receptor clustering. Here, we describe the production of full-length antibody immunotoxins using self-splicing split inteins. To this end, the short (11 amino acids) N-terminal intein part of the artificially designed split intein M86, a derivative of the Ssp DnaB intein, was recombinantly fused to the heavy chain of trastuzumab, a human epidermal growth factor receptor 2 (HER2) receptor targeting antibody and to a nanobody-Fc fusion targeting the HER1 receptor, respectively. Both antibodies were produced in Expi293F cells. The longer C-terminal counterpart of the intein was genetically fused to the protein toxins gelonin or Pseudomonas Exotoxin A, respectively, and expressed in E. coli via fusion to maltose binding protein. Using optimized in vitro splicing conditions, we were able to generate a set of specific and potent immunotoxins with IC50 values in the mid- to subpicomolar range.
中文翻译:

使用分裂内含子通过蛋白质连接产生有效的抗 HER1/2 免疫毒素
细胞靶向蛋白毒素在旨在增加治疗窗和减少全身毒性的癌症治疗中引起了越来越多的兴趣。由于由受体结合部分和细胞杀伤部分组成的免疫毒素的重组表达因其在真核生产宿主中的高毒性而受到阻碍,因此大多数应用依赖于在细菌宿主中重组生产由抗体片段和蛋白质毒素组成的融合蛋白例如大肠杆菌( E. coli )。这些融合体通常缺乏完整抗体的有益特性,例如延长的血清半衰期或通过受体聚集进行有效的内吞摄取。在这里,我们描述了使用自剪接分裂内含肽生产全长抗体免疫毒素。为此,将人工设计的分裂内含肽 M86( Ssp DnaB 内含肽的衍生物)的短(11 个氨基酸) N端内含肽部分重组融合至人表皮生长因子受体 2 曲妥珠单抗的重链(分别针对靶向 HER2) 受体的抗体和靶向 HER1 受体的纳米抗体-Fc 融合体。两种抗体均在 Expi293F 细胞中产生。内含肽的较长 C 端对应物分别与蛋白质毒素 gelonin 或假单胞菌外毒素 A 进行基因融合,并通过与麦芽糖结合蛋白融合在大肠杆菌中表达。使用优化的体外剪接条件,我们能够产生一组特异性且有效的免疫毒素,其IC 50值在中皮摩尔至亚皮摩尔范围内。
更新日期:2018-06-19
中文翻译:

使用分裂内含子通过蛋白质连接产生有效的抗 HER1/2 免疫毒素
细胞靶向蛋白毒素在旨在增加治疗窗和减少全身毒性的癌症治疗中引起了越来越多的兴趣。由于由受体结合部分和细胞杀伤部分组成的免疫毒素的重组表达因其在真核生产宿主中的高毒性而受到阻碍,因此大多数应用依赖于在细菌宿主中重组生产由抗体片段和蛋白质毒素组成的融合蛋白例如大肠杆菌( E. coli )。这些融合体通常缺乏完整抗体的有益特性,例如延长的血清半衰期或通过受体聚集进行有效的内吞摄取。在这里,我们描述了使用自剪接分裂内含肽生产全长抗体免疫毒素。为此,将人工设计的分裂内含肽 M86( Ssp DnaB 内含肽的衍生物)的短(11 个氨基酸) N端内含肽部分重组融合至人表皮生长因子受体 2 曲妥珠单抗的重链(分别针对靶向 HER2) 受体的抗体和靶向 HER1 受体的纳米抗体-Fc 融合体。两种抗体均在 Expi293F 细胞中产生。内含肽的较长 C 端对应物分别与蛋白质毒素 gelonin 或假单胞菌外毒素 A 进行基因融合,并通过与麦芽糖结合蛋白融合在大肠杆菌中表达。使用优化的体外剪接条件,我们能够产生一组特异性且有效的免疫毒素,其IC 50值在中皮摩尔至亚皮摩尔范围内。









































 京公网安备 11010802027423号
京公网安备 11010802027423号