当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Augmenting Vaccine Immunogenicity through the Use of Natural Human Anti-rhamnose Antibodies.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-07-02 , DOI: 10.1021/acschembio.8b00312 Md Kamal Hossain 1 , Abhishek Vartak 2 , Partha Karmakar 2 , Steven J Sucheck 2 , Katherine A Wall 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-07-02 , DOI: 10.1021/acschembio.8b00312 Md Kamal Hossain 1 , Abhishek Vartak 2 , Partha Karmakar 2 , Steven J Sucheck 2 , Katherine A Wall 1
Affiliation
|
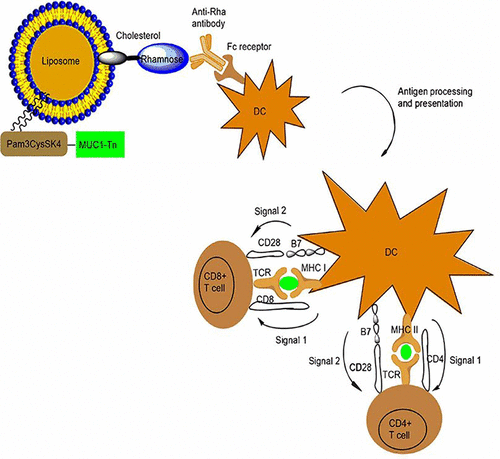
Utilizing natural antibodies to augment vaccine immunogenicity is a promising approach toward cancer immunotherapy. Anti-rhamnose (anti-Rha) antibodies are some of the most common natural anti-carbohydrate antibodies present in human serum. Therefore, rhamnose can be utilized as a targeting moiety for a rhamnose-containing vaccine to prepare an effective vaccine formulation. It was shown previously that anti-Rha antibody generated in mice binds effectively with Rha-conjugated vaccine and is picked up by antigen presenting cells (APCs) through stimulatory Fc receptors. This leads to the effective uptake and processing of antigen and eventually presentation by major histocompatibility complex (MHC) molecules. In this article, we show that natural human anti-Rha antibodies can also be used in a similar mechanism and immunogenicity can be enhanced by targeting Rha-conjugated antigens. In doing so, we have purified human anti-Rha antibodies from human serum using a rhamnose affinity column. In vitro, human anti-Rha antibodies are shown to enhance the uptake of a model antigen, Rha-ovalbumin (Rha-Ova), by APCs. In vivo, they improved the priming of CD4+ T cells to Rha-Ova in comparison to non-anti-Rha human antibodies. Additionally, increased priming of both CD4+ and CD8+ T cells toward the cancer antigen MUC1-Tn was observed in mice that received human anti-Rha antibodies prior to vaccination with a rhamnose-modified MUC1-Tn cancer vaccine. The vaccine conjugate contained Pam3CysSK4, a Toll-like receptor (TLR) agonist linked via copper-free cycloaddition chemistry to a 20-amino-acid glycopeptide derived from the tumor marker MUC-1 containing the tumor-associated carbohydrate antigen α- N-acetyl galactosamine (GalNAc). The primed CD8+ T cells released IFN-γ and killed tumor cells. Therefore, we have confirmed that human anti-Rha antibodies can be effectively utilized as a targeting moiety for making an effective vaccine.
中文翻译:

通过使用天然人类抗鼠李糖抗体来增强疫苗的免疫原性。
利用天然抗体增强疫苗的免疫原性是癌症免疫疗法的一种有前途的方法。抗鼠李糖(anti-Rha)抗体是人血清中存在的一些最常见的天然抗碳水化合物抗体。因此,鼠李糖可以用作含鼠李糖的疫苗的靶向部分以制备有效的疫苗制剂。先前已经证明,在小鼠中产生的抗Rha抗体与结合了Rha的疫苗有效结合,并通过刺激性Fc受体被抗原呈递细胞(APC)拾取。这导致抗原的有效摄取和加工,并最终由主要的组织相容性复合物(MHC)分子呈递。在本文中,我们显示天然人抗Rha抗体也可以类似的机制使用,并且可以通过靶向Rha结合的抗原来增强免疫原性。为此,我们使用鼠李糖亲和柱从人血清中纯化了人抗Rha抗体。在体外,人类抗Rha抗体显示可增强APC对模型抗原Rha-卵清蛋白(Rha-Ova)的吸收。在体内,与非抗Rha人抗体相比,它们改善了CD4 + T细胞对Rha-Ova的引发作用。此外,在鼠李糖修饰的MUC1-Tn癌症疫苗接种之前,在接受人抗Rha抗体的小鼠中观察到CD4 +和CD8 + T细胞对癌症抗原MUC1-Tn的启动增强。疫苗偶联物包含Pam3CysSK4,Toll样受体(TLR)激动剂,通过无铜环加成化学反应与包含肿瘤相关碳水化合物抗原α-N-乙酰半乳糖胺(GalNAc)的肿瘤标记物MUC-1衍生的20个氨基酸的糖肽连接。引发的CD8 + T细胞释放IFN-γ并杀死肿瘤细胞。因此,我们已经证实人抗Rha抗体可以有效地用作制备有效疫苗的靶向部分。
更新日期:2018-06-19
中文翻译:

通过使用天然人类抗鼠李糖抗体来增强疫苗的免疫原性。
利用天然抗体增强疫苗的免疫原性是癌症免疫疗法的一种有前途的方法。抗鼠李糖(anti-Rha)抗体是人血清中存在的一些最常见的天然抗碳水化合物抗体。因此,鼠李糖可以用作含鼠李糖的疫苗的靶向部分以制备有效的疫苗制剂。先前已经证明,在小鼠中产生的抗Rha抗体与结合了Rha的疫苗有效结合,并通过刺激性Fc受体被抗原呈递细胞(APC)拾取。这导致抗原的有效摄取和加工,并最终由主要的组织相容性复合物(MHC)分子呈递。在本文中,我们显示天然人抗Rha抗体也可以类似的机制使用,并且可以通过靶向Rha结合的抗原来增强免疫原性。为此,我们使用鼠李糖亲和柱从人血清中纯化了人抗Rha抗体。在体外,人类抗Rha抗体显示可增强APC对模型抗原Rha-卵清蛋白(Rha-Ova)的吸收。在体内,与非抗Rha人抗体相比,它们改善了CD4 + T细胞对Rha-Ova的引发作用。此外,在鼠李糖修饰的MUC1-Tn癌症疫苗接种之前,在接受人抗Rha抗体的小鼠中观察到CD4 +和CD8 + T细胞对癌症抗原MUC1-Tn的启动增强。疫苗偶联物包含Pam3CysSK4,Toll样受体(TLR)激动剂,通过无铜环加成化学反应与包含肿瘤相关碳水化合物抗原α-N-乙酰半乳糖胺(GalNAc)的肿瘤标记物MUC-1衍生的20个氨基酸的糖肽连接。引发的CD8 + T细胞释放IFN-γ并杀死肿瘤细胞。因此,我们已经证实人抗Rha抗体可以有效地用作制备有效疫苗的靶向部分。










































 京公网安备 11010802027423号
京公网安备 11010802027423号