当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trihalide ionic liquids as non-volatile oxidizing solvents for metals
Green Chemistry ( IF 9.3 ) Pub Date : 2018-06-19 , DOI: 10.1039/c8gc01061g Arne Van den Bossche 1, 2, 3, 4 , Elise De Witte 1, 2, 3, 4 , Wim Dehaen 1, 2, 3, 4 , Koen Binnemans 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2018-06-19 , DOI: 10.1039/c8gc01061g Arne Van den Bossche 1, 2, 3, 4 , Elise De Witte 1, 2, 3, 4 , Wim Dehaen 1, 2, 3, 4 , Koen Binnemans 1, 2, 3, 4
Affiliation
|
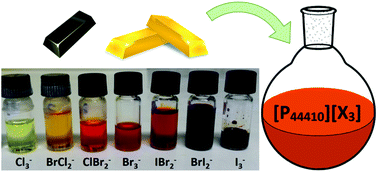
Various ionic liquids containing a trihalide anion [X3]− (X = Cl, Br or I) were synthesized by addition of molecular chlorine, bromine or iodine (X2) to a halide ionic liquid precursor, forming the trihalide anion via a Lewis acid–base reaction. All ionic liquids were characterized by 1H NMR, 13C NMR, 31P NMR, and Raman spectroscopy. Phosphonium trihalide ionic liquids [PR4][X3] (X = Cl, Br or I) were selected because of their low viscosity, low melting point and stability towards chlorine. The melting point, viscosity and density were measured for all ionic liquids. A series of seven trihalide ionic liquids (with anions ranging from trichloride to triiodide), containing both single trihalide and mixed trihalide anions, was prepared for the tributyldecylphosphonium cation. The effect of the trihalide anion on the physical properties of the phosphonium ionic liquids was determined using this series of ionic liquids. Trihalide anions, and polyhalides in general, are strongly oxidizing agents due to their charge deficit with respect to the number of halogen atoms. This strong oxidizing power has been applied to oxidatively dissolve several metals into tributyldecylphosphonium tribromide [P44410][Br3]. A broad range of metals was tested: Fe, Cu, Sb, Co, Zn, In, Ga, Bi, Ge, Sn, Pd, Au, Rh and Pt. With the exception of Pt and Rh, all the metals dissolved in the ionic liquid. The dissolution of Cu in six different trihalide ionic liquids was performed to confirm that these trihalide ionic liquids have similar oxidizing properties to [P44410][Br3]. Due to the negligible vapor pressure of ionic liquids, the entire oxidative dissolution process proceeded in a non-volatile solvent and without the formation of gases. This work can contribute to the development of safe, solvometallurgical methods for oxidative dissolution of metals.
中文翻译:

三卤离子液体作为金属的非挥发性氧化溶剂
通过将分子氯,溴或碘(X 2)添加到卤化物离子液体前体中,通过路易斯形成三卤阴离子[X 3 ] -(X = Cl,Br或I)的各种离子液体。酸碱反应。所有离子液体通过1 H NMR,13 C NMR,31 P NMR和拉曼光谱进行表征。三卤化离子液体[PR 4 ] [X 3选择[X = Cl,Br或I]是因为它们的粘度低,熔点低且对氯具有稳定性。测量所有离子液体的熔点,粘度和密度。为三丁基癸基phosph阳离子制备了一系列七种三卤离子液体(阴离子范围从三氯化物到三碘化物),既包含单个三卤化物又包含混合的三卤化物阴离子。使用这一系列离子液体确定了三卤化物阴离子对the离子液体的物理性能的影响。三卤化物阴离子和多卤化物通常是强氧化剂,这是由于它们相对于卤原子数的电荷不足。这种强大的氧化能力已被用于将几种金属氧化溶解在三溴化三丁基癸基phosph中[P 44410] [Br 3 ]。测试了多种金属:Fe,Cu,Sb,Co,Zn,In,Ga,Bi,Ge,Sn,Pd,Au,Rh和Pt。除Pt和Rh外,所有金属均溶解在离子液体中。进行了将铜溶解在六种不同的三卤化物离子液体中的操作,以确认这些三卤化物离子液体具有与[P 44410 ] [Br 3 ]相似的氧化性能。由于离子液体的蒸气压可忽略不计,因此整个氧化溶解过程都是在非挥发性溶剂中进行的,并且不会形成气体。这项工作可有助于开发用于金属氧化溶解的安全的溶剂冶金方法。
更新日期:2018-07-16
中文翻译:

三卤离子液体作为金属的非挥发性氧化溶剂
通过将分子氯,溴或碘(X 2)添加到卤化物离子液体前体中,通过路易斯形成三卤阴离子[X 3 ] -(X = Cl,Br或I)的各种离子液体。酸碱反应。所有离子液体通过1 H NMR,13 C NMR,31 P NMR和拉曼光谱进行表征。三卤化离子液体[PR 4 ] [X 3选择[X = Cl,Br或I]是因为它们的粘度低,熔点低且对氯具有稳定性。测量所有离子液体的熔点,粘度和密度。为三丁基癸基phosph阳离子制备了一系列七种三卤离子液体(阴离子范围从三氯化物到三碘化物),既包含单个三卤化物又包含混合的三卤化物阴离子。使用这一系列离子液体确定了三卤化物阴离子对the离子液体的物理性能的影响。三卤化物阴离子和多卤化物通常是强氧化剂,这是由于它们相对于卤原子数的电荷不足。这种强大的氧化能力已被用于将几种金属氧化溶解在三溴化三丁基癸基phosph中[P 44410] [Br 3 ]。测试了多种金属:Fe,Cu,Sb,Co,Zn,In,Ga,Bi,Ge,Sn,Pd,Au,Rh和Pt。除Pt和Rh外,所有金属均溶解在离子液体中。进行了将铜溶解在六种不同的三卤化物离子液体中的操作,以确认这些三卤化物离子液体具有与[P 44410 ] [Br 3 ]相似的氧化性能。由于离子液体的蒸气压可忽略不计,因此整个氧化溶解过程都是在非挥发性溶剂中进行的,并且不会形成气体。这项工作可有助于开发用于金属氧化溶解的安全的溶剂冶金方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号