当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Steric vs. electronic stereocontrol in syndio- or iso-selective ROP of functional chiral β-lactones mediated by achiral yttrium-bisphenolate complexes
Chemical Communications ( IF 4.3 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8cc03842b Romain Ligny 1, 2, 3, 4, 5 , Mikko M. Hänninen 6, 7, 8, 9 , Sophie M. Guillaume 1, 2, 3, 4, 5 , Jean-François Carpentier 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8cc03842b Romain Ligny 1, 2, 3, 4, 5 , Mikko M. Hänninen 6, 7, 8, 9 , Sophie M. Guillaume 1, 2, 3, 4, 5 , Jean-François Carpentier 1, 2, 3, 4, 5
Affiliation
|
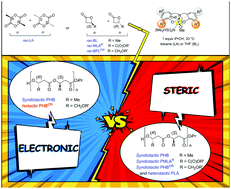
Origins of stereoselectivity in ROP of racemic chiral cyclic esters promoted by achiral yttrium complexes, resulting in the formation of highly heterotactic polylactide, and highly syndiotactic or, more uniquely, highly isotactic poly(3-hydroxybutyrate)s, are discussed. A close interplay between the nature of the cyclic ester, most particularly of the exocyclic functional chain installed on the chiral center of β-lactones, and the ortho-substituents installed on the phenolate rings of the ligand, results in various determining secondary interactions of steric and also electronic nature.
中文翻译:

非手性钇-双酚盐复合物介导的功能性手性β-内酯间位或同位选择性ROP中的 立体与电子立体控制
讨论了由非手性钇配合物促进的外消旋手性环状酯在ROP中的立体选择性,导致形成高度杂规的聚丙交酯,以及高度间同的或更独特的高度等规的聚(3-羟基丁酸酯)。环状酯的性质(尤其是安装在β-内酯的手性中心的环外功能链的性质)与安装在配体的酚盐环上的邻位取代基之间存在密切的相互作用,导致各种确定的空间次级相互作用以及电子性质。
更新日期:2018-06-19
中文翻译:

非手性钇-双酚盐复合物介导的功能性手性β-内酯间位或同位选择性ROP中的 立体与电子立体控制
讨论了由非手性钇配合物促进的外消旋手性环状酯在ROP中的立体选择性,导致形成高度杂规的聚丙交酯,以及高度间同的或更独特的高度等规的聚(3-羟基丁酸酯)。环状酯的性质(尤其是安装在β-内酯的手性中心的环外功能链的性质)与安装在配体的酚盐环上的邻位取代基之间存在密切的相互作用,导致各种确定的空间次级相互作用以及电子性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号