当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(Salen)Mn(iii)-catalyzed chemoselective acylazidation of olefins†
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8sc01882k Liang Zhang 1 , Shuya Liu 1 , Zhiguo Zhao 1 , Hongmei Su 1 , Jingcheng Hao 1 , Yao Wang 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1039/c8sc01882k Liang Zhang 1 , Shuya Liu 1 , Zhiguo Zhao 1 , Hongmei Su 1 , Jingcheng Hao 1 , Yao Wang 1
Affiliation
|
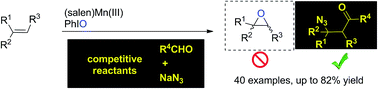
We describe a (salen)Mn(III)-catalyzed three-component reaction of aldehydes, olefins, and sodium azide for the installation of two useful groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N3) into the double bond. Traditionally, (salen)Mn(III) in conjunction with iodosobenzene is a classical catalysis system for epoxidation of olefins. Owing to the highly competitive oxygenation approaches, it is a true challenge to establish a distinct strategy for the exploration of new olefin transformations based on this (salen)Mn(III) catalysis system. Herein, the key to this (salen)Mn(III)-catalyzed acylazidation of olefins was the rational application of the distinct reactivity of oxomanganese(V) species which is capable of abstracting a hydrogen atom from a substrate C–H bond. This chemoselective reaction occurred in a precisely designed reaction sequence and tolerates complex molecular structures.
O and N3) into the double bond. Traditionally, (salen)Mn(III) in conjunction with iodosobenzene is a classical catalysis system for epoxidation of olefins. Owing to the highly competitive oxygenation approaches, it is a true challenge to establish a distinct strategy for the exploration of new olefin transformations based on this (salen)Mn(III) catalysis system. Herein, the key to this (salen)Mn(III)-catalyzed acylazidation of olefins was the rational application of the distinct reactivity of oxomanganese(V) species which is capable of abstracting a hydrogen atom from a substrate C–H bond. This chemoselective reaction occurred in a precisely designed reaction sequence and tolerates complex molecular structures.
中文翻译:

(Salen)Mn(iii) 催化的烯烃化学选择性酰叠氮化†
我们描述了一个 (salen)Mn( III ) 催化的醛、烯烃和叠氮化钠的三组分反应,用于将两个有用的基团(![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO 和 N 3)安装到双键中。传统上,(salen)Mn( III ) 与碘代苯结合是烯烃环氧化的经典催化体系。由于具有高度竞争性的氧化方法,基于这种 (salen)Mn( III ) 催化系统建立独特的探索新烯烃转化的策略是一个真正的挑战。在此,这种 (salen)Mn( III ) 催化的烯烃酰叠氮化反应的关键是合理应用氧锰( V ) 的独特反应性。) 能够从底物 C-H 键中提取氢原子的物质。这种化学选择性反应发生在精确设计的反应序列中,并且可以容忍复杂的分子结构。
CO 和 N 3)安装到双键中。传统上,(salen)Mn( III ) 与碘代苯结合是烯烃环氧化的经典催化体系。由于具有高度竞争性的氧化方法,基于这种 (salen)Mn( III ) 催化系统建立独特的探索新烯烃转化的策略是一个真正的挑战。在此,这种 (salen)Mn( III ) 催化的烯烃酰叠氮化反应的关键是合理应用氧锰( V ) 的独特反应性。) 能够从底物 C-H 键中提取氢原子的物质。这种化学选择性反应发生在精确设计的反应序列中,并且可以容忍复杂的分子结构。
更新日期:2018-06-19
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N3) into the double bond. Traditionally, (salen)Mn(III) in conjunction with iodosobenzene is a classical catalysis system for epoxidation of olefins. Owing to the highly competitive oxygenation approaches, it is a true challenge to establish a distinct strategy for the exploration of new olefin transformations based on this (salen)Mn(III) catalysis system. Herein, the key to this (salen)Mn(III)-catalyzed acylazidation of olefins was the rational application of the distinct reactivity of oxomanganese(V) species which is capable of abstracting a hydrogen atom from a substrate C–H bond. This chemoselective reaction occurred in a precisely designed reaction sequence and tolerates complex molecular structures.
O and N3) into the double bond. Traditionally, (salen)Mn(III) in conjunction with iodosobenzene is a classical catalysis system for epoxidation of olefins. Owing to the highly competitive oxygenation approaches, it is a true challenge to establish a distinct strategy for the exploration of new olefin transformations based on this (salen)Mn(III) catalysis system. Herein, the key to this (salen)Mn(III)-catalyzed acylazidation of olefins was the rational application of the distinct reactivity of oxomanganese(V) species which is capable of abstracting a hydrogen atom from a substrate C–H bond. This chemoselective reaction occurred in a precisely designed reaction sequence and tolerates complex molecular structures.
中文翻译:

(Salen)Mn(iii) 催化的烯烃化学选择性酰叠氮化†
我们描述了一个 (salen)Mn( III ) 催化的醛、烯烃和叠氮化钠的三组分反应,用于将两个有用的基团(
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO 和 N 3)安装到双键中。传统上,(salen)Mn( III ) 与碘代苯结合是烯烃环氧化的经典催化体系。由于具有高度竞争性的氧化方法,基于这种 (salen)Mn( III ) 催化系统建立独特的探索新烯烃转化的策略是一个真正的挑战。在此,这种 (salen)Mn( III ) 催化的烯烃酰叠氮化反应的关键是合理应用氧锰( V ) 的独特反应性。) 能够从底物 C-H 键中提取氢原子的物质。这种化学选择性反应发生在精确设计的反应序列中,并且可以容忍复杂的分子结构。
CO 和 N 3)安装到双键中。传统上,(salen)Mn( III ) 与碘代苯结合是烯烃环氧化的经典催化体系。由于具有高度竞争性的氧化方法,基于这种 (salen)Mn( III ) 催化系统建立独特的探索新烯烃转化的策略是一个真正的挑战。在此,这种 (salen)Mn( III ) 催化的烯烃酰叠氮化反应的关键是合理应用氧锰( V ) 的独特反应性。) 能够从底物 C-H 键中提取氢原子的物质。这种化学选择性反应发生在精确设计的反应序列中,并且可以容忍复杂的分子结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号