当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Water in the Stability of Wild-type and Mutant Insulin Dimers
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-03 , DOI: 10.1021/acs.jpcb.8b04448 Shampa Raghunathan 1 , Krystel El Hage 1 , Jasmine L. Desmond 1 , Lixian Zhang 1 , Markus Meuwly 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-03 , DOI: 10.1021/acs.jpcb.8b04448 Shampa Raghunathan 1 , Krystel El Hage 1 , Jasmine L. Desmond 1 , Lixian Zhang 1 , Markus Meuwly 1
Affiliation
|
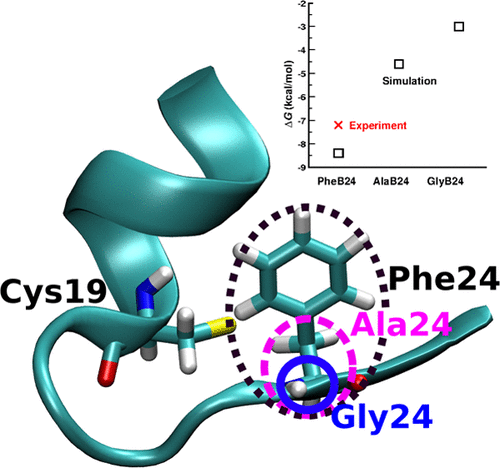
Insulin dimerization and aggregation play important roles in the endogenous delivery of the hormone. One of the important residues at the insulin dimer interface is PheB24, which is an invariant aromatic anchor that packs toward its own monomer inside a hydrophobic cavity formed by ValB12, LeuB15, TyrB16, CysB19, and TyrB26. Using molecular dynamics and free-energy simulations within explicit solvent, the structural and dynamical consequences of mutations of Phe at position B24 to glycine (Gly), alanine (Ala), and d-Ala and the des-PheB25 variant are quantified. Consistent with experiments, it is found that the Gly and Ala modifications lead to insulin dimers with reduced stability by 4 and 5 kcal/mol from thermodynamic integration and 4 and 8 kcal/mol from results using molecular mechanics-generalized Born surface area, respectively. Given the experimental difficulties to quantify the thermodynamic stability of modified insulin dimers, such computations provide a valuable complement. Interestingly, the Gly mutant exists as a strongly and a weakly interacting dimer. Analysis of the molecular dynamics simulations shows that this can be explained by water molecules that replace direct monomer–monomer H-bonding contacts at the dimerization interface involving residues B24 to B26. It is concluded that such solvent molecules play an essential role and must be included in future insulin dimerization studies.
中文翻译:

水在野生型和突变型胰岛素二聚体稳定性中的作用
胰岛素二聚化和聚集在激素的内源性传递中起重要作用。胰岛素二聚体界面上的重要残基之一是Phe B24,它是不变的芳香族锚点,在Val B12,Leu B15,Tyr B16,Cys B19和Tyr B26形成的疏水腔内向自身单体堆积。使用显式溶剂中的分子动力学和自由能模拟,B24位的Phe突变为甘氨酸(Gly),丙氨酸(Ala)和d的结构和动力学后果定量-Ala和des-PheB25变体。与实验一致,发现Gly和Ala修饰导致胰岛素二聚体的热力学积分稳定性降低了4 kcal / mol和5 kcal / mol,而使用分子力学广义Born表面积的结果分别降低了4 kcal / mol和8 kcal / mol。鉴于实验上难以量化修饰的胰岛素二聚体的热力学稳定性,这种计算提供了有价值的补充。有趣的是,Gly突变体以强相互作用和弱相互作用的二聚体形式存在。对分子动力学模拟的分析表明,这可以用水分子代替,该水分子在涉及残基B24至B26的二聚化界面处取代直接的单体-单体H键接触。
更新日期:2018-07-04
中文翻译:

水在野生型和突变型胰岛素二聚体稳定性中的作用
胰岛素二聚化和聚集在激素的内源性传递中起重要作用。胰岛素二聚体界面上的重要残基之一是Phe B24,它是不变的芳香族锚点,在Val B12,Leu B15,Tyr B16,Cys B19和Tyr B26形成的疏水腔内向自身单体堆积。使用显式溶剂中的分子动力学和自由能模拟,B24位的Phe突变为甘氨酸(Gly),丙氨酸(Ala)和d的结构和动力学后果定量-Ala和des-PheB25变体。与实验一致,发现Gly和Ala修饰导致胰岛素二聚体的热力学积分稳定性降低了4 kcal / mol和5 kcal / mol,而使用分子力学广义Born表面积的结果分别降低了4 kcal / mol和8 kcal / mol。鉴于实验上难以量化修饰的胰岛素二聚体的热力学稳定性,这种计算提供了有价值的补充。有趣的是,Gly突变体以强相互作用和弱相互作用的二聚体形式存在。对分子动力学模拟的分析表明,这可以用水分子代替,该水分子在涉及残基B24至B26的二聚化界面处取代直接的单体-单体H键接触。










































 京公网安备 11010802027423号
京公网安备 11010802027423号