Cellular Signalling ( IF 4.4 ) Pub Date : 2018-04-24 , DOI: 10.1016/j.cellsig.2018.04.005 A.F.M. Tariqul Islam , Haicen Yue , Margarethakay Scavello , Pearce Haldeman , Wouter-Jan Rappel , Pascale G. Charest
|
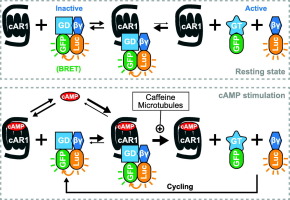
To study the dynamics and mechanisms controlling activation of the heterotrimeric G protein Gα2βγ in Dictyostelium in response to stimulation by the chemoattractant cyclic AMP (cAMP), we monitored the G protein subunit interaction in live cells using bioluminescence resonance energy transfer (BRET). We found that cAMP induces the cAR1-mediated dissociation of the G protein subunits to a similar extent in both undifferentiated and differentiated cells, suggesting that only a small number of cAR1 (as expressed in undifferentiated cells) is necessary to induce the full activation of Gα2βγ. In addition, we found that treating cells with caffeine increases the potency of cAMP-induced Gα2βγ activation; and that disrupting the microtubule network but not F-actin inhibits the cAMP-induced dissociation of Gα2βγ. Thus, microtubules are necessary for efficient cAR1-mediated activation of the heterotrimeric G protein. Finally, kinetics analyses of Gα2βγ subunit dissociation induced by different cAMP concentrations indicate that there are two distinct rates at which the heterotrimeric G protein subunits dissociate when cells are stimulated with cAMP concentrations above 500 nM versus only one rate at lower cAMP concentrations. Quantitative modeling suggests that the kinetics profile of Gα2βγ subunit dissociation results from the presence of both uncoupled and G protein pre-coupled cAR1 that have differential affinities for cAMP and, consequently, induce G protein subunit dissociation through different rates. We suggest that these different signaling kinetic profiles may play an important role in initial chemoattractant gradient sensing.
中文翻译:

通过BRET监测的活Dictyostelium细胞中cAMP诱导的G蛋白亚单位解离揭示了两种激活率,咖啡因的积极作用和微管的潜在作用
为了研究动力学和机制控制的异源三聚G蛋白的Gα2βγ激活粘菌为响应化学引诱剂环AMP(cAMP)的刺激,我们使用生物发光共振能量转移(BRET)监测了活细胞中G蛋白亚基的相互作用。我们发现cAMP在未分化和分化细胞中诱导cAR1介导的G蛋白亚基解离的程度相似,这表明仅少量cAR1(如在未分化细胞中表达)对于诱导Gα2βγ的完全活化是必需的。 。此外,我们发现用咖啡因处理细胞可提高cAMP诱导的Gα2βγ激活的能力;并且破坏微管网络而不破坏F-肌动蛋白抑制了cAMP诱导的Gα2βγ的解离。因此,微管是有效的cAR1介导的异三聚体G蛋白激活所必需的。最后,由不同cAMP浓度引起的Gα2βγ亚基解离的动力学分析表明,当cAMP浓度高于500 nM刺激细胞时,异三聚体G蛋白亚基的解离有两种不同的速率,而在较低cAMP浓度下仅刺激一种。定量建模表明,Gα2βγ亚基解离的动力学特征是由于存在未偶联的cAR1和G蛋白预偶联的cAR1,它们对cAMP具有不同的亲和力,因此通过不同的速率诱导G蛋白亚基的解离。我们建议这些不同的信号动力学配置文件可能在初始化学引力梯度感测中发挥重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号