Cellular Signalling ( IF 4.4 ) Pub Date : 2018-04-17 , DOI: 10.1016/j.cellsig.2018.04.004 Emma Harper , Keith D. Rochfort , Hannah Forde , Colin Davenport , Diarmuid Smith , Philip M. Cummins
|
Background
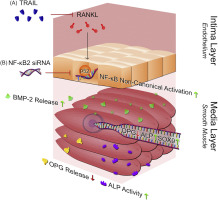
The intimal endothelium is known to condition the underlying medial smooth muscle cell (SMC) layer of the vessel wall, and is highly responsive to receptor-activator of nuclear factor-κB ligand (RANKL) and tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), pro-calcific and anti-calcific agents, respectively. In this paper, we tested the hypothesis that RANKL-induced activation of endothelial NF-κB signalling is essential for pro-calcific activation of the underlying SMCs.
Methods
For these studies, human aortic endothelial and smooth muscle cell mono-cultures (HAECs, HASMCs) were treated with RANKL (0–25 ng/ml ± 5 ng/ml TRAIL) for 72 h. Non-contact transwell HAEC:HASMC co-cultures were also employed in which the luminal HAECs were treated with RANKL (± 5 ng/ml TRAIL), followed by analysis of pro-calcific markers in the underlying subluminal HASMCs.
Results
Treatment of either HAECs or HASMCs with RANKL activated the non-canonical NF-κB/p52 and canonical NF-κB/p65 pathways in both cell types. In RANKL ± TRAIL-treated HAECs, recombinant TRAIL, previously demonstrated by our group to strongly attenuate the pro-calcific signalling effects of RANKL, was shown to specifically block the RANKL-mediated activation of non-canonical NF-κB/p52, clearly pointing to the mechanistic relevance of this specific pathway to RANKL function within endothelial cells. In a final series of HAEC:HASMC transwell co-culture experiments, RANKL treatment of HAECs that had been genetically silenced (via siRNA) for the NF-κB2 gene (the molecular forerunner to NF-κB/p52 generation) exhibited strongly attenuated pro-calcific activation of underlying HASMCs relative to scrambled siRNA controls.
Summary
These in vitro observations provide valuable mechanistic insights into how RANKL may potentially act upon endothelial cells through activation of the alternative NF-κB pathway to alter endothelial paracrine signalling and elicit pro-calcific responses within underlying vascular smooth muscle cells.
中文翻译:

RANKL对血管内皮细胞中非规范性NF-κB/ p52途径的激活在共培养的平滑肌细胞中引起钙化信号转导
背景
已知内膜内皮可调节血管壁的下方内侧平滑肌细胞(SMC)层,并且对核因子-κB配体(RANKL)的受体激活剂和肿瘤坏死因子相关的凋亡诱导配体( TRAIL),促钙剂和抗钙剂。在本文中,我们测试了以下假设,即RANKL诱导的内皮NF-κB信号传导对于基础钙化细胞的钙化激活至关重要。
方法
对于这些研究,将人类主动脉内皮和平滑肌细胞单培养物(HAEC,HASMC)用RANKL(0–25 ng / ml±5 ng / ml TRAIL)处理72小时。还使用了非接触式跨孔HAEC:HASMC共培养,其中腔内HAEC用RANKL(±5 ng / ml TRAIL)处理,然后在下面的腔内HASMC中分析钙化前标志物。
结果
用RANKL治疗HAEC或HASMC会激活两种细胞类型中的非经典NF-κB/ p52和经典NF-κB/ p65途径。在由RANKL±TRAIL处理的HAEC中,我们小组先前证明的重组TRAIL能强烈减弱RANKL的钙化信号转导作用,它被证明可以特异性地阻断RANKL介导的非规范NF-κB/ p52的激活,这一点很明显与内皮细胞中该特定途径与RANKL功能的机制相关。在最后的HAEC:HASMC Transwell共培养实验系列中,RANKL对已通过基因沉默(通过siRNA)抑制NF-κB2基因(NF-κB/ p52产生的先驱分子)的HAEC表现出强烈的减毒作用。相对于加扰的siRNA对照的潜在HASMC的钙化激活。
概括
这些体外观察为RANKL如何通过激活替代性NF-κB途径改变内皮旁分泌信号并引起潜在的血管平滑肌细胞内钙化反应提供了有价值的机制见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号