Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Modeling of Nitric Acid Dissociation Function of Acidity and Temperature
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acsomega.8b00302 Yannis Ziouane 1 , Gilles Leturcq 2
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acsomega.8b00302 Yannis Ziouane 1 , Gilles Leturcq 2
Affiliation
|
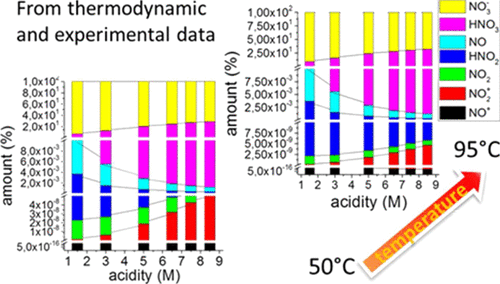
Nitric acid is widely used for many applications. However, because of the large number of nitrogen species and the fact that for concentrations greater than 10–2 mol·L–1, its dissociation is incomplete in an aqueous medium, nitric media are very complex to describe especially in temperature. Using data from the literature and some nitrous acid analyses, it was possible to determine the balance sheet of all different aqueous nitrogen species for a total nitric acidity ranging from 1.5 to 8.5 mol·L–1 and for temperature ranging from 50 to 95 °C with the assumption that the use of a refrigerant column makes gaseous species negligible. For this, it was necessary to determine the ionic, molecular, and solvent activity coefficients and then solve an eight equation system with nine unknowns by determining one unknown experimentally.
中文翻译:

酸度和温度下硝酸离解作用的新模型
硝酸广泛用于许多应用。然而,由于大量的氮物种的,事实上,对于浓度大于10 -2摩尔· -1,其解离是不完整的在含水介质中,硝酸媒体是非常复杂的描述特别是在温度。利用文献数据和亚硝酸分析,可以确定所有不同含水氮物种的资产负债表,其总硝酸度范围为1.5至8.5mol·L –1假设使用制冷剂塔可以使气态物质忽略不计,且温度范围为50至95°C。为此,有必要确定离子,分子和溶剂活度系数,然后通过实验确定一个未知数来求解具有九个未知数的八个方程组。
更新日期:2018-06-19
中文翻译:

酸度和温度下硝酸离解作用的新模型
硝酸广泛用于许多应用。然而,由于大量的氮物种的,事实上,对于浓度大于10 -2摩尔· -1,其解离是不完整的在含水介质中,硝酸媒体是非常复杂的描述特别是在温度。利用文献数据和亚硝酸分析,可以确定所有不同含水氮物种的资产负债表,其总硝酸度范围为1.5至8.5mol·L –1假设使用制冷剂塔可以使气态物质忽略不计,且温度范围为50至95°C。为此,有必要确定离子,分子和溶剂活度系数,然后通过实验确定一个未知数来求解具有九个未知数的八个方程组。











































 京公网安备 11010802027423号
京公网安备 11010802027423号