当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Control of α-Lactalbumin Aggregation by Modulation of Temperature and Concentration of Calcium and Cysteine
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acs.jafc.8b01172 Line R. Nielsen 1, 2 , Søren B. Nielsen 1 , Zichen Zhao 2 , Karsten Olsen 2 , Jacob H. Nielsen 1 , Marianne N. Lund 2, 3
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-06-19 00:00:00 , DOI: 10.1021/acs.jafc.8b01172 Line R. Nielsen 1, 2 , Søren B. Nielsen 1 , Zichen Zhao 2 , Karsten Olsen 2 , Jacob H. Nielsen 1 , Marianne N. Lund 2, 3
Affiliation
|
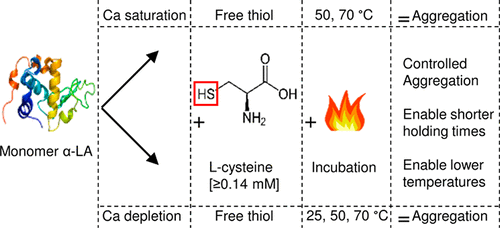
The effect of free cysteine (in different concentrations) on the thermal aggregation of calcium-saturated (Ca-sat) and -depleted (Ca-dep) α-lactalbumin (α-LA) was investigated at 25, 50, and 70 °C. The temperatures chosen were below the denaturation temperature (Td) of Ca-dep and Ca-sat α-LA (25 °C), above the Td of Ca-dep α-LA and below that of Ca-sat α-LA (50 °C), and above the Td of Ca-sat α-LA (70 °C). Size-exclusion chromatography coupled to multiangle light scattering showed that no aggregation or only minor aggregation was obtained at the investigated temperatures for both Ca-dep and Ca-sat α-LA even at extended holding times. Aggregates of Ca-sat α-LA were larger than those developed for Ca-dep α-LA. The addition of cysteine, a low-molecular-mass free thiol, resulted in increased aggregation of both Ca-sat and Ca-dep α-LA. Comparisons of SDS-PAGE run under reducing and nonreducing conditions showed that the formed cross-links were primarily disulfide bonds, but Western blots also showed small contributions from dityrosine cross-link formation. The aggregation kinetics related to monomer loss during heat treatment were determined by RP-UPLC and showed that the addition of cysteine increased the rate of aggregation. The activation energies for Ca-dep α-LA with 0.35 and 0.7 mM cysteine were found to be 59 ± 1 and 46 ± 4 kJ/mol, respectively, which showed that less energy was needed for the enhanced thermal aggregation of α-LA when the cysteine concentration was increased. This study showed that it was possible to control the aggregation size of α-LA by manipulating the incubation temperature and the cysteine concentration.
中文翻译:

通过调节温度以及钙和半胱氨酸的浓度来控制α-乳清蛋白聚集
在25、50和70°C下研究了游离半胱氨酸(不同浓度)对饱和钙(Ca-sat)和贫钙(Ca-dep)α-乳清蛋白(α-LA)的热聚集的影响。选择的温度为低于变性温度(Ť d的Ca-DEP和Ca-SATα-LA(25℃),以上所述的)Ť d的Ca-DEPα-LA的和低于的钙饱和α-LA (50°C),且高于T dCa-satα-LA(70°C)。与多角度光散射耦合的尺寸排阻色谱法表明,即使在延长的保温时间下,对于所研究的Ca-dep和Ca-satα-LA而言,均未获得聚集或仅发生少量聚集。Ca-satα-LA的聚集体大于为Ca-depα-LA形成的聚集体。半胱氨酸(一种低分子质量的游离硫醇)的添加导致Ca-sat和Ca-depα-LA的聚集增加。在还原和非还原条件下进行SDS-PAGE的比较表明,形成的交联主要是二硫键,但Western印迹也显示二氢酪氨酸交联形成的贡献很小。通过RP-UPLC测定与热处理过程中单体损失有关的聚集动力学,结果表明添加半胱氨酸可提高聚集速率。发现具有0.35和0.7 mM半胱氨酸的Ca-depα-LA的活化能分别为59±1和46±4 kJ / mol,这表明当α-LA增强热聚集时所需的能量更少。半胱氨酸浓度增加。这项研究表明,可以通过控制孵育温度和半胱氨酸浓度来控制α-LA的聚集大小。
更新日期:2018-06-19
中文翻译:

通过调节温度以及钙和半胱氨酸的浓度来控制α-乳清蛋白聚集
在25、50和70°C下研究了游离半胱氨酸(不同浓度)对饱和钙(Ca-sat)和贫钙(Ca-dep)α-乳清蛋白(α-LA)的热聚集的影响。选择的温度为低于变性温度(Ť d的Ca-DEP和Ca-SATα-LA(25℃),以上所述的)Ť d的Ca-DEPα-LA的和低于的钙饱和α-LA (50°C),且高于T dCa-satα-LA(70°C)。与多角度光散射耦合的尺寸排阻色谱法表明,即使在延长的保温时间下,对于所研究的Ca-dep和Ca-satα-LA而言,均未获得聚集或仅发生少量聚集。Ca-satα-LA的聚集体大于为Ca-depα-LA形成的聚集体。半胱氨酸(一种低分子质量的游离硫醇)的添加导致Ca-sat和Ca-depα-LA的聚集增加。在还原和非还原条件下进行SDS-PAGE的比较表明,形成的交联主要是二硫键,但Western印迹也显示二氢酪氨酸交联形成的贡献很小。通过RP-UPLC测定与热处理过程中单体损失有关的聚集动力学,结果表明添加半胱氨酸可提高聚集速率。发现具有0.35和0.7 mM半胱氨酸的Ca-depα-LA的活化能分别为59±1和46±4 kJ / mol,这表明当α-LA增强热聚集时所需的能量更少。半胱氨酸浓度增加。这项研究表明,可以通过控制孵育温度和半胱氨酸浓度来控制α-LA的聚集大小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号