当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Substituted Benzenesulfonamides Incorporating Bi/Tricyclic Moieties Act as Potent and Isoform-Selective Carbonic Anhydrase II/IX Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00670 Silvia Salerno 1 , Elisabetta Barresi 1 , Giorgio Amendola 2 , Emanuela Berrino 3 , Ciro Milite 4 , Anna Maria Marini 1 , Federico Da Settimo 1 , Ettore Novellino 5 , Claudiu T Supuran 3 , Sandro Cosconati 2 , Sabrina Taliani 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00670 Silvia Salerno 1 , Elisabetta Barresi 1 , Giorgio Amendola 2 , Emanuela Berrino 3 , Ciro Milite 4 , Anna Maria Marini 1 , Federico Da Settimo 1 , Ettore Novellino 5 , Claudiu T Supuran 3 , Sandro Cosconati 2 , Sabrina Taliani 1
Affiliation
|
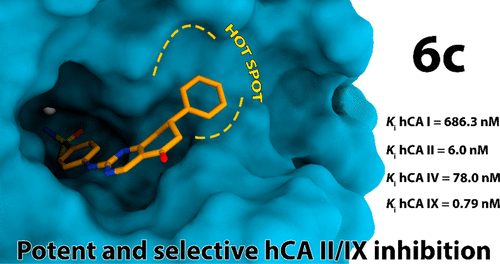
As a part of our efforts to expand chemical diversity in the carbonic anhydrases inhibitors (CAIs), three small series of polyheterocyclic compounds (4–6) featuring the primary benzenesulfonamide moiety linked to bi/tricyclic scaffolds were investigated. Highly effective inhibitors against the target tumor-associated hCA IX (low nanomolar/subnanomolar potency levels) showing significant functional selectivity profile toward hCA I, II, and IV isozymes were identified. Molecular docking studies clarified the reasons behind the activity and selectivity of the new compounds.
中文翻译:

包含双/三环部分的 4-取代苯磺酰胺可作为有效的异构体选择性碳酸酐酶 II/IX 抑制剂
作为我们扩大碳酸酐酶抑制剂 (CAI) 化学多样性的努力的一部分,我们研究了三个小系列的多杂环化合物 ( 4 – 6 ),其特征是与双/三环支架连接的伯苯磺酰胺部分。鉴定出针对靶肿瘤相关 hCA IX(低纳摩尔/亚纳摩尔效力水平)的高效抑制剂,对 hCA I、II 和 IV 同工酶表现出显着的功能选择性。分子对接研究阐明了新化合物的活性和选择性背后的原因。
更新日期:2018-06-18
中文翻译:

包含双/三环部分的 4-取代苯磺酰胺可作为有效的异构体选择性碳酸酐酶 II/IX 抑制剂
作为我们扩大碳酸酐酶抑制剂 (CAI) 化学多样性的努力的一部分,我们研究了三个小系列的多杂环化合物 ( 4 – 6 ),其特征是与双/三环支架连接的伯苯磺酰胺部分。鉴定出针对靶肿瘤相关 hCA IX(低纳摩尔/亚纳摩尔效力水平)的高效抑制剂,对 hCA I、II 和 IV 同工酶表现出显着的功能选择性。分子对接研究阐明了新化合物的活性和选择性背后的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号