当前位置:
X-MOL 学术
›
J. Food Drug Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hantzsch pre-column derivatization for simultaneous determination of alendronate sodium and its pharmacopoeial related impurity: Comparative study with synchronous fluorometry using fluorescamine
Journal of Food and Drug Analysis ( IF 2.6 ) Pub Date : 2019-01-01 , DOI: 10.1016/j.jfda.2018.05.009 Amira F El-Yazbi 1 , Eman I El-Kimary 1 , Rasha M Youssef 1
Journal of Food and Drug Analysis ( IF 2.6 ) Pub Date : 2019-01-01 , DOI: 10.1016/j.jfda.2018.05.009 Amira F El-Yazbi 1 , Eman I El-Kimary 1 , Rasha M Youssef 1
Affiliation
|
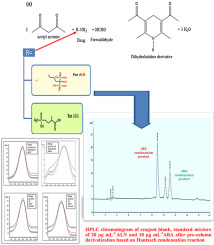
High performance liquid chromatographic (HPLC) method with a pre-column derivatization based on Hantzsch condensation reaction was applied for simultaneous determination of alendronate sodium (ALN) and its main related impurity, 4-Aminobutanoic acid (ABA) at its pharmacopeial limit. The separation of colored condensation products of ALN and ABA were achieved on Agilent Zobrax Eclipse SB-C18 analytical column (250 × 4.6 mm, 5 μm) using a mobile phase composed of acetonitrile-0.1 M acetate buffer, pH 5.0 (15:85, v/v). The flow rate was 1 mL min-1. The detection was carried out at 340 nm using photo-diode array detector. Peak areas were used for the linear regression line in the range of 10-500 and 0.2-40 μg mL-1 for ALN and ABA, respectively. Different conditions for the optimization of the derivatization reactions as well as for the HPLC measurement were studied. The proposed method was validated for linearity, precision, accuracy, specificity and robustness. This method was used to check the purity of ALN in the presence of ABA (related impurity) at the pharmacopeial limit (0.5%). For comparison purpose, another method was proposed which involves synchronous fluorescence measurement after ALN reaction with fluorescamine. In this method, the third derivative synchronous spectra were estimated as peak to peak measurement from 339 to 370 nm for ALN determination with LOD and LOQ of 24 and 73 ng mL-1, respectively, showing very high sensitivity. Both methods have been applied for determination of the alendronate sodium (ALN) in bulk and pharmaceutical preparations without interference of additives in tablets or oral solution.
中文翻译:

Hantzsch 柱前衍生同时测定阿仑膦酸钠及其药典相关杂质:使用荧光胺的同步荧光法的比较研究
采用基于 Hantzsch 缩合反应的柱前衍生高效液相色谱 (HPLC) 方法同时测定阿仑膦酸钠 (ALN) 及其主要相关杂质 4-氨基丁酸 (ABA) 的药典限量。使用 Agilent Zobrax Eclipse SB-C18 分析柱 (250 × 4.6 mm, 5 μm) 分离 ALN 和 ABA 的有色缩合产物,流动相由 0.1 M 乙腈缓冲液组成,pH 5.0 (15:85, v/v)。流速为 1 mL min-1。使用光电二极管阵列检测器在 340 nm 处进行检测。峰面积分别用于 ALN 和 ABA 在 10-500 和 0.2-40 μg mL-1 范围内的线性回归线。研究了优化衍生反应以及 HPLC 测量的不同条件。对所提出的方法的线性、精密度、准确度、特异性和稳健性进行了验证。该方法用于在药典限度 (0.5%) 存在 ABA(相关杂质)的情况下检查 ALN 的纯度。为了比较的目的,提出了另一种方法,该方法涉及在ALN与荧光胺反应后进行同步荧光测量。在该方法中,三阶导数同步光谱估计为 339 至 370 nm 的峰间测量值,用于 ALN 测定,LOD 和 LOQ 分别为 24 和 73 ng mL-1,显示出非常高的灵敏度。
更新日期:2019-01-01
中文翻译:

Hantzsch 柱前衍生同时测定阿仑膦酸钠及其药典相关杂质:使用荧光胺的同步荧光法的比较研究
采用基于 Hantzsch 缩合反应的柱前衍生高效液相色谱 (HPLC) 方法同时测定阿仑膦酸钠 (ALN) 及其主要相关杂质 4-氨基丁酸 (ABA) 的药典限量。使用 Agilent Zobrax Eclipse SB-C18 分析柱 (250 × 4.6 mm, 5 μm) 分离 ALN 和 ABA 的有色缩合产物,流动相由 0.1 M 乙腈缓冲液组成,pH 5.0 (15:85, v/v)。流速为 1 mL min-1。使用光电二极管阵列检测器在 340 nm 处进行检测。峰面积分别用于 ALN 和 ABA 在 10-500 和 0.2-40 μg mL-1 范围内的线性回归线。研究了优化衍生反应以及 HPLC 测量的不同条件。对所提出的方法的线性、精密度、准确度、特异性和稳健性进行了验证。该方法用于在药典限度 (0.5%) 存在 ABA(相关杂质)的情况下检查 ALN 的纯度。为了比较的目的,提出了另一种方法,该方法涉及在ALN与荧光胺反应后进行同步荧光测量。在该方法中,三阶导数同步光谱估计为 339 至 370 nm 的峰间测量值,用于 ALN 测定,LOD 和 LOQ 分别为 24 和 73 ng mL-1,显示出非常高的灵敏度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号