当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
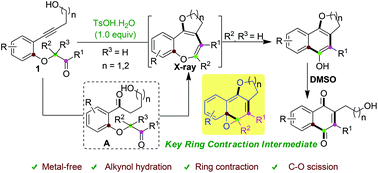
p-TsOH promoted synthesis of benzo-fused O-heterocycles from alkynols via ring contraction and C–O scission strategy
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-18 , DOI: 10.1039/c8gc00749g Gopal Chandru Senadi,Jeh-Jeng Wang
Green Chemistry ( IF 9.8 ) Pub Date : 2018-06-18 , DOI: 10.1039/c8gc00749g Gopal Chandru Senadi,Jeh-Jeng Wang
|
Here, we report the first divergent synthesis of benzo-fused O-heterocycles by p-toluene sulfonic acid promoted cascade reactions involving alkyne hydration, double cyclization, ring contraction and C–O bond cleavage from alkynols. The reaction mechanism was validated by obtaining the X-ray structure of fused benzo[b]oxepine intermediates. Moreover, some of the obtained derivatives could be transformed into 2,3-disubstituted naphthoquinones as important structural motifs in natural products and bioactive molecules.
中文翻译:

p -TsOH通过环收缩和C–O断裂策略促进了炔烃合成苯并稠合的O-杂环
在这里,我们报道了由对甲苯磺酸促进苯并稠合的O杂环的首次发散合成,该反应促进了级联反应,涉及炔烃水合,双环化,环收缩和炔醇裂解C-O键。通过获得熔融的苯并[ b ]氧杂环庚烷中间体的X射线结构来验证反应机理。而且,一些获得的衍生物可以转化为2,3-二取代的萘醌,作为天然产物和生物活性分子中的重要结构基序。
更新日期:2018-07-30
中文翻译:

p -TsOH通过环收缩和C–O断裂策略促进了炔烃合成苯并稠合的O-杂环
在这里,我们报道了由对甲苯磺酸促进苯并稠合的O杂环的首次发散合成,该反应促进了级联反应,涉及炔烃水合,双环化,环收缩和炔醇裂解C-O键。通过获得熔融的苯并[ b ]氧杂环庚烷中间体的X射线结构来验证反应机理。而且,一些获得的衍生物可以转化为2,3-二取代的萘醌,作为天然产物和生物活性分子中的重要结构基序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号