当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the chemoselectivities between carbonyl and hydroxyl groups in the Rh(ii)–azavinyl carbene involved reactions†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1039/c8cy00899j Yuanzheng Lv 1, 2, 3, 4, 5 , Abosede Adejoke Ogunlana 1, 2, 3, 4, 5 , Hongli Li 1, 2, 3, 4, 5 , Dafang Gao 1, 2, 3, 4, 5 , Chenli Wang 1, 2, 3, 4, 5 , Xiaoguang Bao 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1039/c8cy00899j Yuanzheng Lv 1, 2, 3, 4, 5 , Abosede Adejoke Ogunlana 1, 2, 3, 4, 5 , Hongli Li 1, 2, 3, 4, 5 , Dafang Gao 1, 2, 3, 4, 5 , Chenli Wang 1, 2, 3, 4, 5 , Xiaoguang Bao 1, 2, 3, 4, 5
Affiliation
|
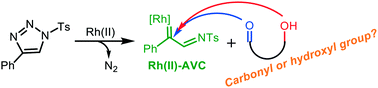
Computational studies were carried out to understand the chemoselectivities between carbonyl and hydroxyl groups in the Rh(II)–azavinyl carbene (Rh(II)–AVC) intermediate involved reactions. For the Rh(II)-catalyzed tandem reaction of 1-sulfonyl-1,2,3-triazoles with salicylaldehydes to produce 2,5-epoxy-1,4-benzoxazepines, the nucleophilic addition of the carbonyl group to the carbenoid of Rh(II)–AVC occurs more readily than the nucleophilic addition of the hydroxyl group and C–H group of the phenyl ring, affording a formal [3 + 2] cycloaddition oxazoline intermediate. A detailed mechanistic pathway for the subsequent conversion of the oxazoline intermediate to the final product was revealed. In contrast, for the Rh-catalyzed reactions of 1-sulfonyl-1,2,3-triazoles with 4-hydroxyacetophenone and with α,β-unsaturated cyclic ketones bearing an aliphatic hydroxyl group, the nucleophilic addition of the hydroxyl group to the carbenoid of Rh(II)–AVC is more favorable than the nucleophilic addition of the carbonyl group. The main factors responsible for the preferred nucleophilic addition of carbonyl/hydroxyl groups to the carbenoid of Rh(II)–AVC were discussed.
中文翻译:

了解Rh(ii)-氮杂乙烯基卡宾中羰基和羟基之间的化学选择性涉及反应†
进行了计算研究,以了解Rh(II)-氮杂乙烯基卡宾(Rh(II)-AVC)中间反应中羰基和羟基之间的化学选择性。对于Rh(II)催化的1-磺酰基-1,2,3-三唑与水杨醛的串联反应,生成2,5-环氧-1,4-苯并氮杂ze庚烯,羰基的亲核加成反应是Rh的类胡萝卜素(二)-AVC比苯环的羟基和CH的亲核加成更容易发生,从而提供了正式的[3 + 2]环加成恶唑啉中间体。揭示了恶唑啉中间体随后转化为最终产物的详细机理。相反,对于1-磺酰基-1,2,3-三唑与4-羟基苯乙酮以及带有脂肪族羟基的α,β-不饱和环状酮的Rh催化反应,羟基向类胡萝卜素的亲核加成Rh(II)–AVC的取代度比羰基的亲核加成更有利。讨论了将羰基/羟基优选亲核加成到Rh(II)-AVC的类胡萝卜素上的主要因素。
更新日期:2018-06-18
中文翻译:

了解Rh(ii)-氮杂乙烯基卡宾中羰基和羟基之间的化学选择性涉及反应†
进行了计算研究,以了解Rh(II)-氮杂乙烯基卡宾(Rh(II)-AVC)中间反应中羰基和羟基之间的化学选择性。对于Rh(II)催化的1-磺酰基-1,2,3-三唑与水杨醛的串联反应,生成2,5-环氧-1,4-苯并氮杂ze庚烯,羰基的亲核加成反应是Rh的类胡萝卜素(二)-AVC比苯环的羟基和CH的亲核加成更容易发生,从而提供了正式的[3 + 2]环加成恶唑啉中间体。揭示了恶唑啉中间体随后转化为最终产物的详细机理。相反,对于1-磺酰基-1,2,3-三唑与4-羟基苯乙酮以及带有脂肪族羟基的α,β-不饱和环状酮的Rh催化反应,羟基向类胡萝卜素的亲核加成Rh(II)–AVC的取代度比羰基的亲核加成更有利。讨论了将羰基/羟基优选亲核加成到Rh(II)-AVC的类胡萝卜素上的主要因素。









































 京公网安备 11010802027423号
京公网安备 11010802027423号