当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aryl bis-sulfonamides bind to the active site of a homotrimeric isoprenoid biosynthesis enzyme IspF and extract the essential divalent metal cation cofactor†
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1039/c8sc00814k Katharina Root 1 , Konstantin Barylyuk 1 , Anatol Schwab 1 , Jonas Thelemann 1 , Boris Illarionov 2 , Julie G Geist 1 , Tobias Gräwert 2 , Adelbert Bacher 3 , Markus Fischer 2 , François Diederich 1 , Renato Zenobi 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1039/c8sc00814k Katharina Root 1 , Konstantin Barylyuk 1 , Anatol Schwab 1 , Jonas Thelemann 1 , Boris Illarionov 2 , Julie G Geist 1 , Tobias Gräwert 2 , Adelbert Bacher 3 , Markus Fischer 2 , François Diederich 1 , Renato Zenobi 1
Affiliation
|
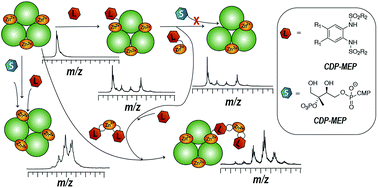
Characterizing the mode of action of non-covalent inhibitors in multisubunit enzymes often presents a great challenge. Most of the conventionally used methods are based on ensemble measurements of protein–ligand binding in bulk solution. They often fail to accurately describe multiple binding processes occurring in such systems. Native electrospray ionization mass spectrometry (ESI-MS) of intact protein complexes is a direct, label-free approach that can render the entire distribution of ligand-bound states in multimeric protein complexes. Here we apply native ESI-MS to comprehensively characterize the isoprenoid biosynthesis enzyme IspF from Arabidopsis thaliana, an example of a homomeric protein complex with multiple binding sites for several types of ligands, including a metal cofactor and a synthetic inhibitor. While standard biophysical techniques failed to reveal the mode of action of recently discovered aryl-sulfonamide-based inhibitors of AtIspF, direct native ESI-MS titrations of the protein with the ligands and ligand competition assays allowed us to accurately capture the solution-phase protein–ligand binding equilibria in full complexity and detail. Based on these combined with computational modeling, we propose a mechanism of AtIspF inhibition by aryl bis-sulfonamides that involves both the competition with the substrate for the ligand-binding pocket and the extraction of Zn2+ from the enzyme active site. This inhibition mode is therefore mixed competitive and non-competitive, the latter exerting a key inhibitory effect on the enzyme activity. The results of our study deliver a profound insight into the mechanisms of AtIspF action and inhibition, open new perspectives for designing inhibitors of this important drug target, and demonstrate the applicability and value of the native ESI-MS approach for deep analysis of complex biomolecular binding equilibria.
中文翻译:

芳基双磺酰胺与同源三聚类异戊二烯生物合成酶 IspF 的活性位点结合,并提取必需的二价金属阳离子辅因子†
表征多亚基酶中非共价抑制剂的作用模式通常是一个巨大的挑战。大多数常规使用的方法都是基于本体溶液中蛋白质-配体结合的整体测量。他们通常无法准确描述此类系统中发生的多个结合过程。完整蛋白质复合物的天然电喷雾电离质谱 (ESI-MS) 是一种直接、无标记的方法,可以呈现多聚蛋白质复合物中配体结合状态的完整分布。在这里,我们应用天然 ESI-MS 来全面表征来自拟南芥的类异戊二烯生物合成酶 IspF,这是一种同聚蛋白复合物的例子,具有多种配体的多个结合位点,包括金属辅因子和合成抑制剂。虽然标准生物物理技术未能揭示最近发现的基于芳基磺酰胺的At IspF 抑制剂的作用模式,但用配体直接对蛋白质进行天然 ESI-MS 滴定和配体竞争测定使我们能够准确捕获溶液相蛋白质– 完全复杂和详细的配体结合平衡。基于这些与计算模型的结合,我们提出了芳基双磺酰胺抑制At IspF 的机制,该机制涉及与配体结合袋的底物竞争以及从酶活性位点提取 Zn 2+ 。因此,这种抑制模式是竞争性和非竞争性混合的,后者对酶活性发挥关键的抑制作用。 我们的研究结果深入了解了At IspF 的作用和抑制机制,为设计这一重要药物靶点的抑制剂开辟了新的视角,并证明了原生 ESI-MS 方法在复杂生物分子深度分析中的适用性和价值结合平衡。
更新日期:2018-06-18
中文翻译:

芳基双磺酰胺与同源三聚类异戊二烯生物合成酶 IspF 的活性位点结合,并提取必需的二价金属阳离子辅因子†
表征多亚基酶中非共价抑制剂的作用模式通常是一个巨大的挑战。大多数常规使用的方法都是基于本体溶液中蛋白质-配体结合的整体测量。他们通常无法准确描述此类系统中发生的多个结合过程。完整蛋白质复合物的天然电喷雾电离质谱 (ESI-MS) 是一种直接、无标记的方法,可以呈现多聚蛋白质复合物中配体结合状态的完整分布。在这里,我们应用天然 ESI-MS 来全面表征来自拟南芥的类异戊二烯生物合成酶 IspF,这是一种同聚蛋白复合物的例子,具有多种配体的多个结合位点,包括金属辅因子和合成抑制剂。虽然标准生物物理技术未能揭示最近发现的基于芳基磺酰胺的At IspF 抑制剂的作用模式,但用配体直接对蛋白质进行天然 ESI-MS 滴定和配体竞争测定使我们能够准确捕获溶液相蛋白质– 完全复杂和详细的配体结合平衡。基于这些与计算模型的结合,我们提出了芳基双磺酰胺抑制At IspF 的机制,该机制涉及与配体结合袋的底物竞争以及从酶活性位点提取 Zn 2+ 。因此,这种抑制模式是竞争性和非竞争性混合的,后者对酶活性发挥关键的抑制作用。 我们的研究结果深入了解了At IspF 的作用和抑制机制,为设计这一重要药物靶点的抑制剂开辟了新的视角,并证明了原生 ESI-MS 方法在复杂生物分子深度分析中的适用性和价值结合平衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号