当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-assembly of toroidal proteins explored using native mass spectrometry†
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1039/c8sc01379a N. Amy Yewdall 1, 2, 3, 4, 5 , Timothy M. Allison 6, 7, 8, 9 , F. Grant Pearce 1, 2, 3, 4, 5 , Carol V. Robinson 6, 7, 8, 9 , Juliet A. Gerrard 1, 5, 10, 11, 12
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-18 00:00:00 , DOI: 10.1039/c8sc01379a N. Amy Yewdall 1, 2, 3, 4, 5 , Timothy M. Allison 6, 7, 8, 9 , F. Grant Pearce 1, 2, 3, 4, 5 , Carol V. Robinson 6, 7, 8, 9 , Juliet A. Gerrard 1, 5, 10, 11, 12
Affiliation
|
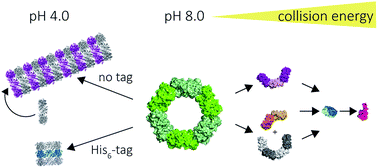
The peroxiredoxins are a well characterised family of toroidal proteins which can self-assemble into a striking array of quaternary structures, including protein nanotubes, making them attractive as building blocks for nanotechnology. Tools to characterise these assemblies are currently scarce. Here, assemblies of peroxiredoxin proteins were examined using native mass spectrometry and complementary solution techniques. We demonstrated unequivocally that tube formation is fully reversible, a useful feature in a molecular switch. Simple assembly of individual toroids was shown to be tunable by pH and the presence of a histidine tag. Collision induced dissociation experiments on peroxiredoxin rings revealed a highly unusual symmetrical disassembly pathway, consistent with the structure disassembling as a hexamer of dimers. This study provides the foundation for the rational design and precise characterisation of peroxiredoxin protein structures where self-assembly can be harnessed as a key feature for applications in nanotechnology.
中文翻译:

使用天然质谱探索环形蛋白质的自组装†
过氧化物酶是环蛋白的一个特征鲜明的家族,可以自组装成惊人的四级结构阵列,包括蛋白质纳米管,使其成为纳米技术的基础。用于表征这些组件的工具目前很少。在这里,使用天然质谱和互补溶液技术检查了过氧化物酶蛋白的装配。我们明确证明了管的形成是完全可逆的,这在分子转换中是有用的功能。显示单个环的简单组装可通过pH值和组氨酸标签的存在进行调节。在过氧化物酶毒素环上的碰撞诱导解离实验揭示了高度不寻常的对称分解途径,与作为二聚体的六聚体分解的结构一致。
更新日期:2018-06-18
中文翻译:

使用天然质谱探索环形蛋白质的自组装†
过氧化物酶是环蛋白的一个特征鲜明的家族,可以自组装成惊人的四级结构阵列,包括蛋白质纳米管,使其成为纳米技术的基础。用于表征这些组件的工具目前很少。在这里,使用天然质谱和互补溶液技术检查了过氧化物酶蛋白的装配。我们明确证明了管的形成是完全可逆的,这在分子转换中是有用的功能。显示单个环的简单组装可通过pH值和组氨酸标签的存在进行调节。在过氧化物酶毒素环上的碰撞诱导解离实验揭示了高度不寻常的对称分解途径,与作为二聚体的六聚体分解的结构一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号