当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibria for 2-Propanol Dehydration through Extractive Distillation Using Mixed Solvent of Ethylene Glycol and Choline Chloride
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-18 , DOI: 10.1021/acs.jced.8b00162 Lianzhong Zhang 1 , Mingyang Lan 1 , Xuejiao Wu 1 , Yichi Zhang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-18 , DOI: 10.1021/acs.jced.8b00162 Lianzhong Zhang 1 , Mingyang Lan 1 , Xuejiao Wu 1 , Yichi Zhang 1
Affiliation
|
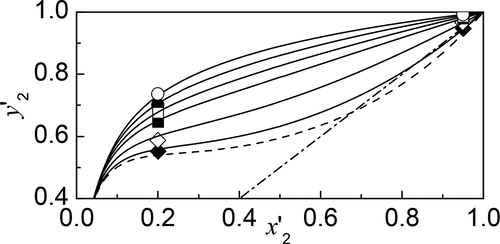
The mixed solvent of ethylene glycol (EG) and choline chloride (ChCl) was tested as an entrainer for 2-propanol dehydration by extractive distillation. Isobaric vapor–liquid equilibrium (VLE) data were measured at 101.3 kPa for the quaternary system water + 2-propanol + EG + ChCl. The NRTL equation was used for the modeling of the quaternary VLE. Mean absolute deviations were 0.26 K and 0.0036 for equilibrium temperature and vapor mole fraction of 2-propanol, respectively. As compared with EG alone, the existence of ChCl in the solvent significantly decreases the activity coefficient of water and increases the activity coefficient of 2-propanol, both enhancing the relative volatility of 2-propanol to water. The azeotrope of water +2-propanol can be removed with the addition of the deep eutectic mixture EG + ChCl 2:1 at a solvent mass fraction of 0.157. The mixed solvent may be readily applied in extractive distillation of 2-propanol and water, replacing the frequently used mixed solvent of ethylene glycol and potassium acetate.
中文翻译:

使用乙二醇和氯化胆碱的混合溶剂通过萃取蒸馏进行2-丙醇脱水的汽液平衡
测试了乙二醇(EG)和氯化胆碱(ChCl)的混合溶剂作为通过萃取蒸馏进行2-丙醇脱水的夹带剂。对于四元系统水+ 2-丙醇+ EG + ChCl,在101.3 kPa下测量了等压气液平衡(VLE)数据。NRTL方程用于四元VLE的建模。2-丙醇的平衡温度和蒸气摩尔分数的平均绝对偏差分别为0.26 K和0.0036。与单独的EG相比,溶剂中ChCl的存在显着降低了水的活度系数并增加了2-丙醇的活度系数,均增强了2-丙醇对水的相对挥发性。可以通过添加溶剂质量分数为0的深共晶混合物EG + ChCl 2:1除去水+ 2-丙醇的共沸物。
更新日期:2018-06-18
中文翻译:

使用乙二醇和氯化胆碱的混合溶剂通过萃取蒸馏进行2-丙醇脱水的汽液平衡
测试了乙二醇(EG)和氯化胆碱(ChCl)的混合溶剂作为通过萃取蒸馏进行2-丙醇脱水的夹带剂。对于四元系统水+ 2-丙醇+ EG + ChCl,在101.3 kPa下测量了等压气液平衡(VLE)数据。NRTL方程用于四元VLE的建模。2-丙醇的平衡温度和蒸气摩尔分数的平均绝对偏差分别为0.26 K和0.0036。与单独的EG相比,溶剂中ChCl的存在显着降低了水的活度系数并增加了2-丙醇的活度系数,均增强了2-丙醇对水的相对挥发性。可以通过添加溶剂质量分数为0的深共晶混合物EG + ChCl 2:1除去水+ 2-丙醇的共沸物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号