Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-06-18 , DOI: 10.1016/j.tetlet.2018.06.035 Kailasam Saravana Mani , Balasubramanian Murugesapandian , Werner Kaminsky , Subramaniam Parameswaran Rajendran
|
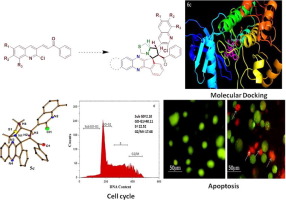
The 1,3-dipolar cycloaddition reactions of azomethine ylide generated in situ from indeno quinoxaline and thiazolidine-2-carboxylic acid to a series of quinoline bearing dipolarophile afforded novel spiro indeno-quinoxaline pyrrolo thiazoles in quantitative yields. The newly synthesized compounds were characterized using different spectroscopic techniques. Furthermore, the molecular structure of compound 5c was confirmed by single crystal X-ray crystallography. The synthesized compounds were screened for their in vitro antioxidant activity and in vitro cytotoxic activity against breast cancer cell line MCF-7 and adenocarcinomic cancer cell line A-549. Compound containing more electron donors in quinoline site were found to be more potent with good IC50 values.
中文翻译:

对映体选择性合成螺-茚并[1,2- b ]喹喔啉吡咯并噻唑类化合物作为抗氧化剂和抗增殖剂
从茚并喹喔啉和噻唑烷-2-羧酸原位生成的偶氮甲碱的1,3-偶极环加成反应与一系列带有喹啉的双极性亲核试剂定量反应得到了新型螺茚并喹喔啉吡咯并噻唑。使用不同的光谱技术对新合成的化合物进行了表征。此外,通过单晶X射线晶体学确认了化合物5c的分子结构。筛选合成的化合物对乳腺癌细胞系MCF-7和腺癌癌细胞系A-549的体外抗氧化活性和体外细胞毒性活性。发现在喹啉位点上包含更多电子供体的化合物具有更佳的IC 50值,而且更有效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号