PLOS ONE ( IF 2.9 ) Pub Date : 2018-06-15 , DOI: 10.1371/journal.pone.0198990 Kalyanasundaram Subramanian 1 , Artur Góra 2 , Ruud Spruijt 1 , Karolina Mitusińska 2, 3 , Maria Suarez-Diez 1 , Vitor Martins Dos Santos 1 , Peter J Schaap 1
|
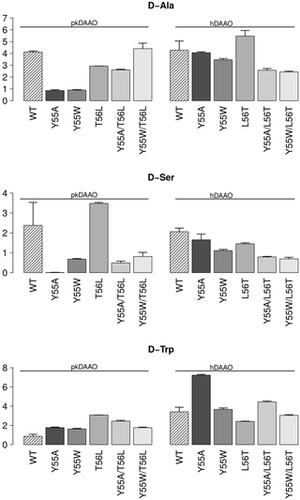
D-amino acid oxidase (DAAO) degrades D-amino acids to produce α-ketoacids, hydrogen peroxide and ammonia. DAAO has often been investigated and engineered for industrial and clinical applications. We combined information from literature with a detailed analysis of the structure to engineer mammalian DAAOs. The structural analysis was complemented with molecular dynamics simulations to characterize solvent accessibility and product release mechanisms. We identified non-obvious residues located on the loops on the border between the active site and the secondary binding pocket essential for pig and human DAAO substrate specificity and activity. We engineered DAAOs by mutating such critical residues and characterised the biochemical activity of the resulting variants. The results highlight the importance of the selected residues in modulating substrate specificity, product egress and enzyme activity, suggesting further steps of DAAO re-engineering towards desired clinical and industrial applications.
中文翻译:

通过促进溶剂进入调节 D-氨基酸氧化酶 (DAAO) 底物特异性
D-氨基酸氧化酶 (DAAO) 降解 D-氨基酸,产生 α-酮酸、过氧化氢和氨。 DAAO 经常被研究和设计用于工业和临床应用。我们将文献中的信息与结构的详细分析结合起来,设计了哺乳动物 DAAO。结构分析辅以分子动力学模拟,以表征溶剂可及性和产品释放机制。我们鉴定出位于活性位点和对猪和人类 DAAO 底物特异性和活性至关重要的次级结合袋之间的边界环上的不明显残基。我们通过突变这些关键残基来设计 DAAO,并表征所得变体的生化活性。结果强调了所选残基在调节底物特异性、产物排出和酶活性方面的重要性,表明 DAAO 重新设计的进一步步骤可实现所需的临床和工业应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号