Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Analysis of the Carbon-Encapsulated Lithium Iron Phosphate Nanochains and Their High-Temperature Conductivity Profiles
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acsomega.8b00527 K. P. Abhilash , P. Christopher Selvin 1 , B. Nalini 2 , Hui Xia , Stefan Adams , M. V. Reddy
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acsomega.8b00527 K. P. Abhilash , P. Christopher Selvin 1 , B. Nalini 2 , Hui Xia , Stefan Adams , M. V. Reddy
Affiliation
|
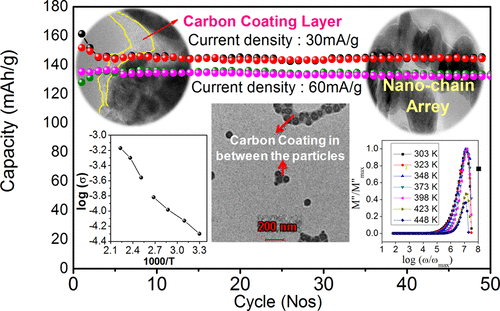
Carbon-encapsulated LiFePO4 (LFP) nanochains were prepared as a cathode material for lithium batteries by sol–gel method using citric acid as the carbon source. The prepared LFP/C material is characterized by structural, morphological, and electrochemical characterization. LFP/C shows an orthorhombic olivine structure with “Pnma” space group having an average particle size of 50 nm. The uniform distribution of LFP particles coated by the carbon matrix as a nanochain array has been analyzed by scanning electron microscopy and transmission electron microscopy analysis of the sample. The electrochemical performance of the LFP/C nanochain has been analyzed using galvanostatic cycling, cyclic voltammetry, and impedance analysis of the assembled batteries. The sol–gel-derived LFP/C nanochain exhibits better capacity and electrochemical reversibility in line with the literature results. The high-temperature conductivity profile of the sample has been recorded from room temperature to 473 K using impedance analysis of the sample. The transport dynamics have been analyzed using the dielectric and modulus spectra of the sample. A maximum conductivity up to 6.74 × 10–4 S cm–1 has been obtained for the samples at higher temperature (448 K). The nucleation and growth at higher temperature act as factors to facilitate the intermediate phase existence in the LiFePO4 sample in which the phase change that occurs above 400 K gives irreversible electrochemical changes in the LFP/C samples.
中文翻译:

碳包裹的磷酸铁锂锂纳米链的电化学分析及其高温电导曲线
采用柠檬酸作为碳源,通过溶胶-凝胶法制备了碳封装的LiFePO 4(LFP)纳米链作为锂电池的正极材料。制备的LFP / C材料的特征在于结构,形态和电化学特性。LFP / C显示具有“ Pnma ”的正交斜橄榄石结构平均粒径为50 nm的空间群。已经通过样品的扫描电子显微镜和透射电子显微镜分析来分析被碳基质作为纳米链阵列涂覆的LFP颗粒的均匀分布。LFP / C纳米链的电化学性能已使用恒电流循环,循环伏安法和组装电池的阻抗分析进行了分析。溶胶-凝胶衍生的LFP / C纳米链表现出更好的容量和电化学可逆性,与文献结果一致。使用样品的阻抗分析,已记录了从室温到473 K的样品的高温电导率曲线。使用样品的介电谱和模量谱分析了传输动力学。最大电导率高达6.74×10对于较高温度(448 K)的样品,获得了–4 S cm –1。高温下的成核和生长是促进LiFePO 4样品中存在中间相的因素,其中在400 K以上发生的相变会在LFP / C样品中产生不可逆的电化学变化。
更新日期:2018-06-15
中文翻译:

碳包裹的磷酸铁锂锂纳米链的电化学分析及其高温电导曲线
采用柠檬酸作为碳源,通过溶胶-凝胶法制备了碳封装的LiFePO 4(LFP)纳米链作为锂电池的正极材料。制备的LFP / C材料的特征在于结构,形态和电化学特性。LFP / C显示具有“ Pnma ”的正交斜橄榄石结构平均粒径为50 nm的空间群。已经通过样品的扫描电子显微镜和透射电子显微镜分析来分析被碳基质作为纳米链阵列涂覆的LFP颗粒的均匀分布。LFP / C纳米链的电化学性能已使用恒电流循环,循环伏安法和组装电池的阻抗分析进行了分析。溶胶-凝胶衍生的LFP / C纳米链表现出更好的容量和电化学可逆性,与文献结果一致。使用样品的阻抗分析,已记录了从室温到473 K的样品的高温电导率曲线。使用样品的介电谱和模量谱分析了传输动力学。最大电导率高达6.74×10对于较高温度(448 K)的样品,获得了–4 S cm –1。高温下的成核和生长是促进LiFePO 4样品中存在中间相的因素,其中在400 K以上发生的相变会在LFP / C样品中产生不可逆的电化学变化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号