当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Paradoxically, Most Flexible Ligand Binds Most Entropy-Favored: Intriguing Impact of Ligand Flexibility and Solvation on Drug–Kinase Binding
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-06-16 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00105 Barbara Wienen-Schmidt 1 , Hendrik R. A. Jonker 2 , Tobias Wulsdorf 1 , Hans-Dieter Gerber 1 , Krishna Saxena 2 , Denis Kudlinzki 2 , Sridhar Sreeramulu 2 , Giacomo Parigi 3 , Claudio Luchinat 3 , Andreas Heine 1 , Harald Schwalbe 2 , Gerhard Klebe 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-06-16 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00105 Barbara Wienen-Schmidt 1 , Hendrik R. A. Jonker 2 , Tobias Wulsdorf 1 , Hans-Dieter Gerber 1 , Krishna Saxena 2 , Denis Kudlinzki 2 , Sridhar Sreeramulu 2 , Giacomo Parigi 3 , Claudio Luchinat 3 , Andreas Heine 1 , Harald Schwalbe 2 , Gerhard Klebe 1
Affiliation
|
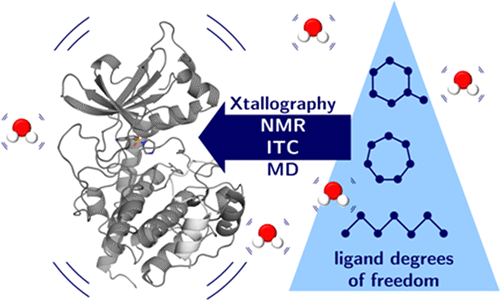
Biophysical parameters can accelerate drug development; e.g., rigid ligands may reduce entropic penalty and improve binding affinity. We studied systematically the impact of ligand rigidification on thermodynamics using a series of fasudil derivatives inhibiting protein kinase A by crystallography, isothermal titration calorimetry, nuclear magnetic resonance, and molecular dynamics simulations. The ligands varied in their internal degrees of freedom but conserve the number of heteroatoms. Counterintuitively, the most flexible ligand displays the entropically most favored binding. As experiment shows, this cannot be explained by higher residual flexibility of ligand, protein, or formed complex nor by a deviating or increased release of water molecules upon complex formation. NMR and crystal structures show no differences in flexibility and water release, although strong ligand-induced adaptations are observed. Instead, the flexible ligand entraps more efficiently water molecules in solution prior to protein binding, and by release of these waters, the favored entropic binding is observed.
中文翻译:

矛盾的是,最灵活的配体结合了最偏爱熵的:配体柔韧性和溶剂化对药物激酶结合的有趣影响
生物物理参数可以加速药物开发;例如,刚性配体可以减少熵损失并改善结合亲和力。我们通过晶体学,等温滴定量热法,核磁共振和分子动力学模拟,系统地研究了使用一系列fasudil衍生物抑制蛋白激酶A的配体刚性对热力学的影响。配体的内部自由度各不相同,但保留了杂原子数。与直觉相反,最灵活的配体表现出熵最有利的结合。如实验所示,这不能解释为配体,蛋白质或形成的复合物具有更高的残留柔韧性,也不能通过形成复合物时水分子的偏离或增加释放来解释。尽管观察到强的配体诱导的适应性,但NMR和晶体结构在柔韧性和水释放方面没有差异。相反,柔性配体可以更有效地将水分子截留在溶液中在蛋白质结合之前,并且通过释放这些水,观察到有利的熵结合。
更新日期:2018-06-16
中文翻译:

矛盾的是,最灵活的配体结合了最偏爱熵的:配体柔韧性和溶剂化对药物激酶结合的有趣影响
生物物理参数可以加速药物开发;例如,刚性配体可以减少熵损失并改善结合亲和力。我们通过晶体学,等温滴定量热法,核磁共振和分子动力学模拟,系统地研究了使用一系列fasudil衍生物抑制蛋白激酶A的配体刚性对热力学的影响。配体的内部自由度各不相同,但保留了杂原子数。与直觉相反,最灵活的配体表现出熵最有利的结合。如实验所示,这不能解释为配体,蛋白质或形成的复合物具有更高的残留柔韧性,也不能通过形成复合物时水分子的偏离或增加释放来解释。尽管观察到强的配体诱导的适应性,但NMR和晶体结构在柔韧性和水释放方面没有差异。相反,柔性配体可以更有效地将水分子截留在溶液中在蛋白质结合之前,并且通过释放这些水,观察到有利的熵结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号