当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective Electrochemical Reduction of Dinitrogen to Ammonia at Ambient Temperature and Pressure over Iron Oxide Catalysts
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-08-10 , DOI: 10.1002/chem.201800535 Xiaoyang Cui 1 , Cheng Tang 1 , Xiao‐Meng Liu 1 , Chen Wang 1 , Wenjun Ma 1 , Qiang Zhang 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-08-10 , DOI: 10.1002/chem.201800535 Xiaoyang Cui 1 , Cheng Tang 1 , Xiao‐Meng Liu 1 , Chen Wang 1 , Wenjun Ma 1 , Qiang Zhang 1
Affiliation
|
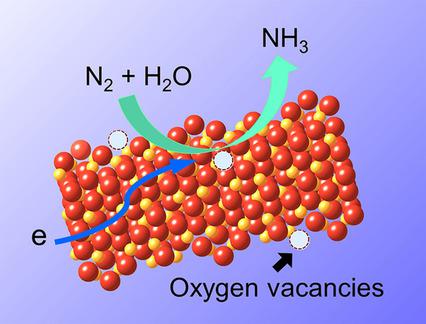
The catalytic conversion of dinitrogen (N2) into ammonia under ambient conditions represents one of the Holy Grails in sustainable chemistry. As a potential alternative to the Haber–Bosch process, the electrochemical reduction of N2 to NH3 is attractive owing to its renewability and flexibility, as well as its sustainability for producing and storing value‐added chemicals from the abundant feedstock of water and nitrogen on earth. However, owing to the kinetically complex and energetically challenging N2 reduction reaction (NRR) process, NRR electrocatalysts with high catalytic activity and high selectivity are rare. In this contribution, as a proof‐of‐concept, we demonstrate that both the NH3 yield and faradaic efficiency (FE) under ambient conditions can be improved by modification of the hematite nanostructure surface. Introducing more oxygen vacancies to the hematite surface renders an improved performance in NRR, which leads to an average NH3 production rate of 0.46 μg h−1 cm−2 and an NH3 FE of 6.04 % at −0.9 V vs. Ag/AgCl in 0.10 m KOH electrolyte. The durability of the electrochemical system was also investigated. A surprisingly high average NH3 production rate of 1.45 μg h−1 cm−2 and a NH3 FE of 8.28 % were achieved after the first 1 h chronoamperometry test. This is among the highest FEs reported so far for non‐precious‐metal catalysts that use a polymer‐electrolyte‐membrane cell and is much higher than the FE of precious‐metal catalysts (e.g., Ru/C) under comparable reaction conditions. However, the NH3 yield and the FE dropped to 0.29 μg h−1 cm−2 and 2.74 %, respectively, after 16 h of chronoamperometry tests, which indicates poor durability of the system. Our results demonstrate the important role that the surface states of transition‐metal oxides have in promoting electrocatalytic NRR under ambient conditions. This work may spur interest towards the rational design of electrocatalysts as well as electrochemical systems for NRR, with emphasis on the issue of stability.
中文翻译:

氧化铁催化剂在常温常压下高选择性电化学还原二氮为氨
在环境条件下,将二氮(N 2)催化转化为氨代表了可持续化学领域的“圣杯”。作为Haber-Bosch工艺的潜在替代品,N 2电化学还原为NH 3具有吸引力,这归因于其可更新性和灵活性以及其从丰富的水和氮原料生产和存储增值化学品的可持续性在地球上。然而,由于动力学复杂且在能量上具有挑战性的N 2还原反应(NRR)过程,具有高催化活性和高选择性的NRR电催化剂很少见。在此贡献中,作为概念验证,我们证明了NH 3通过修饰赤铁矿纳米结构表面可以提高环境条件下的产率和法拉第效率(FE)。向赤铁矿表面引入更多的氧空位可改善NRR性能,从而在-0.9 V相对于Ag / AgCl的情况下,平均NH 3生产率为0.46μgh -1 cm -2,而NH 3 FE为6.04%。在0.10 m KOH电解液中 还研究了电化学系统的耐久性。1.45μgh -1 cm -2和NH 3的平均NH 3产生率高得惊人在最初的1小时计时电流法测试之后,FE达到8.28%。这是迄今为止报道的使用聚合物电解质膜电池的非贵金属催化剂的最高FEs之一,并且在可比的反应条件下,其远高于贵金属催化剂的FE(例如Ru / C)。但是,NH 3收率和FE下降至0.29μgh -1 cm -2。在计时电流法测试16小时后,分别为2.74%和2.74%,这表明系统的耐用性较差。我们的结果证明了过渡金属氧化物的表面状态在环境条件下对促进电催化NRR的重要作用。这项工作可能激发人们对合理设计电催化剂以及用于NRR的电化学系统的兴趣,重点是稳定性问题。
更新日期:2018-08-10
中文翻译:

氧化铁催化剂在常温常压下高选择性电化学还原二氮为氨
在环境条件下,将二氮(N 2)催化转化为氨代表了可持续化学领域的“圣杯”。作为Haber-Bosch工艺的潜在替代品,N 2电化学还原为NH 3具有吸引力,这归因于其可更新性和灵活性以及其从丰富的水和氮原料生产和存储增值化学品的可持续性在地球上。然而,由于动力学复杂且在能量上具有挑战性的N 2还原反应(NRR)过程,具有高催化活性和高选择性的NRR电催化剂很少见。在此贡献中,作为概念验证,我们证明了NH 3通过修饰赤铁矿纳米结构表面可以提高环境条件下的产率和法拉第效率(FE)。向赤铁矿表面引入更多的氧空位可改善NRR性能,从而在-0.9 V相对于Ag / AgCl的情况下,平均NH 3生产率为0.46μgh -1 cm -2,而NH 3 FE为6.04%。在0.10 m KOH电解液中 还研究了电化学系统的耐久性。1.45μgh -1 cm -2和NH 3的平均NH 3产生率高得惊人在最初的1小时计时电流法测试之后,FE达到8.28%。这是迄今为止报道的使用聚合物电解质膜电池的非贵金属催化剂的最高FEs之一,并且在可比的反应条件下,其远高于贵金属催化剂的FE(例如Ru / C)。但是,NH 3收率和FE下降至0.29μgh -1 cm -2。在计时电流法测试16小时后,分别为2.74%和2.74%,这表明系统的耐用性较差。我们的结果证明了过渡金属氧化物的表面状态在环境条件下对促进电催化NRR的重要作用。这项工作可能激发人们对合理设计电催化剂以及用于NRR的电化学系统的兴趣,重点是稳定性问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号