Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transformation mechanism of sodium during pyrolysis of Zhundong coal
Fuel ( IF 6.7 ) Pub Date : 2018-12-01 , DOI: 10.1016/j.fuel.2018.06.038 Lianfei Xu , Hui Liu , Deng Zhao , Qingxi Cao , Jihui Gao , Shaohua Wu
Fuel ( IF 6.7 ) Pub Date : 2018-12-01 , DOI: 10.1016/j.fuel.2018.06.038 Lianfei Xu , Hui Liu , Deng Zhao , Qingxi Cao , Jihui Gao , Shaohua Wu
|
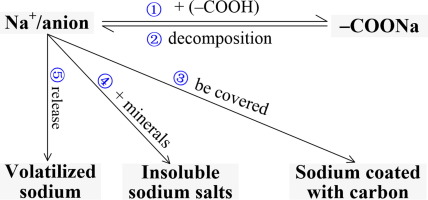
Abstract The modes in which Na occurs play an important role in its release and catalytic effects during thermal utilization of Zhundong coal. The modes of Na transform with increasing temperature during pyrolysis, but the transformation mechanism remains unclear. In this study, an acid-washed coal was used as a substrate to eliminate the influence of Na inherently present in the raw coal. Na2SO4, NaCl, NaHCO3, and sodium carboxylate were loaded onto the acid-washed coal by impregnation or ion exchange. Pyrolysis of the sodium-loaded coals was conducted in a fixed-bed reactor. Transformation of Na in chars was studied over the temperature range 300–900 °C. Interconversion of H2O- and CH3COONH4-soluble sodium occurred below 400 °C. CH3COONH4-soluble sodium mainly transformed to H2O-soluble sodium at 400–600 °C. The transformation of Na from the H2O-soluble to insoluble form occurred above 600 °C. The transformation of Na related to its initial form in the coal. Transformation routes and mechanisms of Na are proposed·H2O-soluble sodium can react with COOH to form CH3COONH4-soluble sodium below 400 °C. The decomposition of CH3COONH4-soluble sodium occurred at temperatures lower than 600 °C. H2O-soluble sodium may combine with minerals or be coated by the carbon matrix to form insoluble sodium above 600 °C. Na that volatilized above 600 °C was mainly in the H2O-soluble form.
中文翻译:

准东煤热解过程中钠的转化机理
摘要 准东煤热利用过程中Na的发生方式对其释放和催化作用起着重要作用。Na在热解过程中随着温度的升高而发生转变,但转变机制尚不清楚。在这项研究中,酸洗煤被用作基质,以消除原煤中固有的 Na 的影响。Na2SO4、NaCl、NaHCO3 和羧酸钠通过浸渍或离子交换加载到酸洗煤上。载钠煤的热解在固定床反应器中进行。在 300–900 °C 的温度范围内研究了碳中 Na 的转化。H2O- 和 CH3COONH4 可溶性钠的相互转化发生在 400 °C 以下。CH3COONH4 可溶性钠主要在 400–600 °C 转化为 H2O 可溶性钠。Na 从 H2O 可溶形式到不溶形式的转变发生在 600 °C 以上。Na 的转化与其在煤中的初始形态有关。提出了Na的转化路线和机理·H2O可溶性钠在400℃以下与COOH反应生成CH3COONH4可溶性钠。CH3COONH4 可溶性钠的分解发生在低于 600 °C 的温度下。H2O 可溶性钠在 600 °C 以上可能与矿物质结合或被碳基质包覆形成不溶性钠。在 600 °C 以上挥发的 Na 主要以 H2O 可溶形式存在。CH3COONH4 可溶性钠的分解发生在低于 600 °C 的温度下。H2O 可溶性钠在 600 °C 以上可能与矿物质结合或被碳基质包覆形成不溶性钠。在 600 °C 以上挥发的 Na 主要以 H2O 可溶形式存在。CH3COONH4 可溶性钠的分解发生在低于 600 °C 的温度下。H2O 可溶性钠在 600 °C 以上可能与矿物质结合或被碳基质包覆形成不溶性钠。在 600 °C 以上挥发的 Na 主要以 H2O 可溶形式存在。
更新日期:2018-12-01
中文翻译:

准东煤热解过程中钠的转化机理
摘要 准东煤热利用过程中Na的发生方式对其释放和催化作用起着重要作用。Na在热解过程中随着温度的升高而发生转变,但转变机制尚不清楚。在这项研究中,酸洗煤被用作基质,以消除原煤中固有的 Na 的影响。Na2SO4、NaCl、NaHCO3 和羧酸钠通过浸渍或离子交换加载到酸洗煤上。载钠煤的热解在固定床反应器中进行。在 300–900 °C 的温度范围内研究了碳中 Na 的转化。H2O- 和 CH3COONH4 可溶性钠的相互转化发生在 400 °C 以下。CH3COONH4 可溶性钠主要在 400–600 °C 转化为 H2O 可溶性钠。Na 从 H2O 可溶形式到不溶形式的转变发生在 600 °C 以上。Na 的转化与其在煤中的初始形态有关。提出了Na的转化路线和机理·H2O可溶性钠在400℃以下与COOH反应生成CH3COONH4可溶性钠。CH3COONH4 可溶性钠的分解发生在低于 600 °C 的温度下。H2O 可溶性钠在 600 °C 以上可能与矿物质结合或被碳基质包覆形成不溶性钠。在 600 °C 以上挥发的 Na 主要以 H2O 可溶形式存在。CH3COONH4 可溶性钠的分解发生在低于 600 °C 的温度下。H2O 可溶性钠在 600 °C 以上可能与矿物质结合或被碳基质包覆形成不溶性钠。在 600 °C 以上挥发的 Na 主要以 H2O 可溶形式存在。CH3COONH4 可溶性钠的分解发生在低于 600 °C 的温度下。H2O 可溶性钠在 600 °C 以上可能与矿物质结合或被碳基质包覆形成不溶性钠。在 600 °C 以上挥发的 Na 主要以 H2O 可溶形式存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号