当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Silylium (R3Si+) Catalyzed Condensative Cyclization for Anhydrosugar Synthesis
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acscatal.8b01666 Youngran Seo 1 , Michel R. Gagné 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acscatal.8b01666 Youngran Seo 1 , Michel R. Gagné 1
Affiliation
|
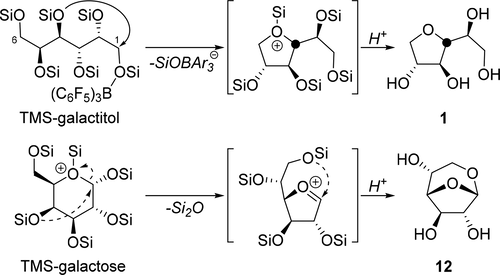
The silylium (Me2EtSi+) catalyzed condensative cyclization of persilylated linear or cyclic sugars has been examined as a selective route to monoanhydro sugars. The silylium could be generated either from the sequential addition of [Ph3C+][B(C6F5)4–] and R3SiH (where Si is the silyl protecting group (either trimethylsilyl (TMS) or Me2EtSi)) or by abstracting a primary silyl ether with the Lewis acid catalyst B(C6F5)3. This latter method forms [R3SiO–B(C6F5)3–] and a silylium-coordinated product. This reactive pair eventually converts to L–B(C6F5)3 (L = THF-like product) and the condensate (R3Si)2O. The L–B(C6F5)3 is a much less potent form of the catalyst. Under the silylium catalyzed conditions, linear sugars (CnOn, n = 5, 6) and biomass-derived tetraols (C6O4) afford a diverse collection of diastereomerically pure THFs in good to excellent yields (50–99%). Persilylated 1,6-anhydrogalactofuranose is provided in a single step from persilylated galactose (85% isolation yield after silyl deprotection) and the corresponding 1,6-anhydroglucoses are available from mono-, di-, tri, and oligo-gluco-saccharides.
中文翻译:

硅(R 3 Si +)催化缩合环化合成脱水糖。
已经研究了甲硅烷基(Me 2 EtSi +)催化的全硅烷基化直链或环状糖的缩合环化反应,作为制备单脱水糖的选择性途径。可以通过依次添加[Ph 3 C + ] [B(C 6 F 5)4 – ]和R 3 SiH(其中Si是甲硅烷基保护基(三甲基甲硅烷基(TMS)或Me 2 EtSi ))或通过路易斯酸催化剂B(C 6 F 5)3提取伯甲硅烷基醚。后一种方法形成[R 3 SiO–B(C 6 F5)3 – ]和甲硅烷基配位产物。该反应性对最终转换到L-B(C 6 ˚F 5)3(L = THF状产物)和冷凝物(R 3 Si)的2 O的L-B(C 6 ˚F 5)3是一个更催化剂的有效形式。在甲硅烷基催化的条件下,直链糖(C n O n,n = 5,6)和生物质衍生的四醇(C 6 O 4)以良好或优异的收率(50–99%)提供了各种非对映体纯的THF。只需一步即可从全硅烷基化的半乳糖中获得全硅烷基化的1,6-脱水半乳糖呋喃糖(甲硅烷基脱保护后的分离产率为85%),相应的1,6-脱水葡萄糖可从单糖,二糖,三糖和寡糖获得。
更新日期:2018-06-15
中文翻译:

硅(R 3 Si +)催化缩合环化合成脱水糖。
已经研究了甲硅烷基(Me 2 EtSi +)催化的全硅烷基化直链或环状糖的缩合环化反应,作为制备单脱水糖的选择性途径。可以通过依次添加[Ph 3 C + ] [B(C 6 F 5)4 – ]和R 3 SiH(其中Si是甲硅烷基保护基(三甲基甲硅烷基(TMS)或Me 2 EtSi ))或通过路易斯酸催化剂B(C 6 F 5)3提取伯甲硅烷基醚。后一种方法形成[R 3 SiO–B(C 6 F5)3 – ]和甲硅烷基配位产物。该反应性对最终转换到L-B(C 6 ˚F 5)3(L = THF状产物)和冷凝物(R 3 Si)的2 O的L-B(C 6 ˚F 5)3是一个更催化剂的有效形式。在甲硅烷基催化的条件下,直链糖(C n O n,n = 5,6)和生物质衍生的四醇(C 6 O 4)以良好或优异的收率(50–99%)提供了各种非对映体纯的THF。只需一步即可从全硅烷基化的半乳糖中获得全硅烷基化的1,6-脱水半乳糖呋喃糖(甲硅烷基脱保护后的分离产率为85%),相应的1,6-脱水葡萄糖可从单糖,二糖,三糖和寡糖获得。



























 京公网安备 11010802027423号
京公网安备 11010802027423号