当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
[OSSO]-Type Iron(III) Complexes for the Low-Pressure Reaction of Carbon Dioxide with Epoxides: Catalytic Activity, Reaction Kinetics, and Computational Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acscatal.8b01695 Francesco Della Monica 1 , Bholanath Maity 2 , Thomas Pehl 3 , Antonio Buonerba 1 , Assunta De Nisi 4 , Magda Monari 4 , Alfonso Grassi 1 , Bernhard Rieger 3 , Luigi Cavallo 2 , Carmine Capacchione 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1021/acscatal.8b01695 Francesco Della Monica 1 , Bholanath Maity 2 , Thomas Pehl 3 , Antonio Buonerba 1 , Assunta De Nisi 4 , Magda Monari 4 , Alfonso Grassi 1 , Bernhard Rieger 3 , Luigi Cavallo 2 , Carmine Capacchione 1
Affiliation
|
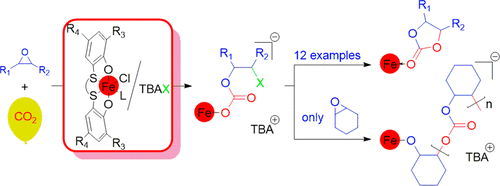
The selective conversion of variously substituted epoxides into the corresponding cyclic carbonates under mild reaction conditions was achieved with mononuclear Fe(III) complexes bearing bis-thioether-diphenolate [OSSO]-type ligands, in combination with tetrabutylammonium bromide (TBAB). For example, propylene carbonate was obtained in 1 h at 35 °C (turnover frequency, TOF = 290 h–1), from propylene oxide and 1 bar of CO2 pressure, using 0.1 mol % of the Fe(III) complex and 0.5 mol % of TBAB. Product divergence is observed only for cyclohexene oxide toward the exclusive formation of the aliphatic polycarbonate (TOF = 165 h–1 at 80 °C and 1 bar of CO2 pressure, using 0.1 mol % of the Fe(III) complex and 0.1 mol % of tetrabutylammonium chloride). Kinetic investigations indicated reaction orders of two and one, with respect to the Fe(III) complex, for the production of propylene carbonate and the poly(cyclohexene carbonate), respectively. The enthalpy and entropy of activation were determined using the Eyring equation [for propylene carbonate: ΔH‡ = 8.4 ± 0.7 kcal/mol and ΔS‡ = −33 ± 3 cal/(mol·K); for poly(cyclohexene carbonate): ΔH‡ = 11.9 ± 0.3 kal/mol and ΔS‡ = −36 ± 2.2 cal/(mol·K)]. Supported by density functional theory based investigations, we propose a mechanistic scenario in which the rate-limiting step is the bimetallic ring opening of the epoxide, in the case of propylene carbonate, and the monometallic insertion of the epoxide in the growing polymer chain, in the case of poly(cyclohexene carbonate).
中文翻译:

用于二氧化碳与环氧化合物低压反应的[OSSO]型铁(III)配合物:催化活性,反应动力学和计算研究
使用带有双硫醚-二酚盐[OSSO]型配体的单核Fe(III)配合物,结合四丁基溴化铵(TBAB),在温和的反应条件下,将各种取代的环氧化物选择性转化为相应的环状碳酸酯。例如,使用0.1 mol%的Fe(III)络合物和0.5 mol%的三氧化二丙烯和1 bar的CO 2压力,在35°C(转换频率,TOF = 290 h –1)下,于1小时内获得碳酸亚丙酯。TBAB的摩尔%。仅对于环己烯氧化物,朝着仅形成脂肪族聚碳酸酯的方向观察到了产品差异(在80°C和1 bar CO 2下,TOF = 165 h –1使用0.1mol%的Fe(III)配合物和0.1mol%的氯化四丁基铵)。动力学研究表明,相对于Fe(III)配合物,碳酸亚丙酯和聚碳酸环己烯的反应顺序分别为2和1。使用Eyring方程[对于碳酸亚丙酯:ΔH ‡ = 8.4±0.7 kcal / mol和ΔS ‡ = -33±3 cal /(mol·K)确定活化的焓和熵。于聚碳酸环己烯酯:ΔH ‡ = 11.9±0.3 kal / mol和ΔS ‡= -36±2.2cal /(mol·K)]。在基于密度泛函理论的研究的支持下,我们提出了一种机械方案,其中限速步骤是在碳酸亚丙酯的情况下环氧化物的双金属开环,以及环氧化物在增长的聚合物链中的单金属插入。聚(碳酸环己烯酯)的情况。
更新日期:2018-06-15
中文翻译:

用于二氧化碳与环氧化合物低压反应的[OSSO]型铁(III)配合物:催化活性,反应动力学和计算研究
使用带有双硫醚-二酚盐[OSSO]型配体的单核Fe(III)配合物,结合四丁基溴化铵(TBAB),在温和的反应条件下,将各种取代的环氧化物选择性转化为相应的环状碳酸酯。例如,使用0.1 mol%的Fe(III)络合物和0.5 mol%的三氧化二丙烯和1 bar的CO 2压力,在35°C(转换频率,TOF = 290 h –1)下,于1小时内获得碳酸亚丙酯。TBAB的摩尔%。仅对于环己烯氧化物,朝着仅形成脂肪族聚碳酸酯的方向观察到了产品差异(在80°C和1 bar CO 2下,TOF = 165 h –1使用0.1mol%的Fe(III)配合物和0.1mol%的氯化四丁基铵)。动力学研究表明,相对于Fe(III)配合物,碳酸亚丙酯和聚碳酸环己烯的反应顺序分别为2和1。使用Eyring方程[对于碳酸亚丙酯:ΔH ‡ = 8.4±0.7 kcal / mol和ΔS ‡ = -33±3 cal /(mol·K)确定活化的焓和熵。于聚碳酸环己烯酯:ΔH ‡ = 11.9±0.3 kal / mol和ΔS ‡= -36±2.2cal /(mol·K)]。在基于密度泛函理论的研究的支持下,我们提出了一种机械方案,其中限速步骤是在碳酸亚丙酯的情况下环氧化物的双金属开环,以及环氧化物在增长的聚合物链中的单金属插入。聚(碳酸环己烯酯)的情况。











































 京公网安备 11010802027423号
京公网安备 11010802027423号