当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity of arynes toward functionalized alkenes: intermolecular Alder-ene vs. addition reactions†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8qo00470f Saswata Gupta 1, 2, 3, 4 , Peipei Xie 5, 6, 7, 8 , Yuanzhi Xia 5, 6, 7, 8 , Daesung Lee 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8qo00470f Saswata Gupta 1, 2, 3, 4 , Peipei Xie 5, 6, 7, 8 , Yuanzhi Xia 5, 6, 7, 8 , Daesung Lee 1, 2, 3, 4
Affiliation
|
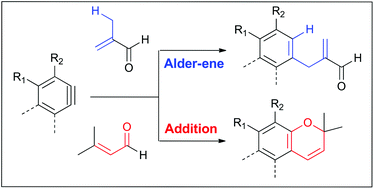
The selectivity between two different manifolds of reactions of arynes reacting with functionalized alkenes is described. Arynes generated from bis-1,3-diynes react with various trisubstituted and 1,1-disubstituted alkenes including methallyl amine, prenyl azide, and methacrylic acid, providing mainly addition products of the polar heteroatom functionalities over the Alder-ene products of the alkene segment. The selectivity, however, intricately depends on the substituent pattern of the alkene. Except for the most reactive 2-propenyl group-containing aldehyde, α,β-unsaturated aldehydes generally participated in an addition reaction, generating chromene derivatives.
中文翻译:

芳烃对官能化烯烃的反应性:分子间的Alder-ene与加成反应†
描述了芳烃与官能化烯烃的反应的两个不同歧管之间的选择性。由双1,3-二炔生成的芳烃与各种三取代和1,1-二取代的烯烃(包括甲基烯丙基胺,异戊烯基叠氮化物和甲基丙烯酸)反应,主要提供极性杂原子官能度的加成产物,而不是烯烃的Alder-ene产物。分割。然而,选择性复杂地取决于烯烃的取代基图案。除了最具反应性的含2-丙烯基的醛外,α,β-不饱和醛通常参与加成反应,生成苯并二氢萘衍生物。
更新日期:2018-06-15
中文翻译:

芳烃对官能化烯烃的反应性:分子间的Alder-ene与加成反应†
描述了芳烃与官能化烯烃的反应的两个不同歧管之间的选择性。由双1,3-二炔生成的芳烃与各种三取代和1,1-二取代的烯烃(包括甲基烯丙基胺,异戊烯基叠氮化物和甲基丙烯酸)反应,主要提供极性杂原子官能度的加成产物,而不是烯烃的Alder-ene产物。分割。然而,选择性复杂地取决于烯烃的取代基图案。除了最具反应性的含2-丙烯基的醛外,α,β-不饱和醛通常参与加成反应,生成苯并二氢萘衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号