Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
User-defined local stimulation of live tissue through a movable microfluidic port†
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8lc00204e Megan A. Catterton 1, 2, 3, 4 , Austin F. Dunn 1, 2, 3, 4 , Rebecca R. Pompano 1, 2, 3, 4, 5
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-06-15 00:00:00 , DOI: 10.1039/c8lc00204e Megan A. Catterton 1, 2, 3, 4 , Austin F. Dunn 1, 2, 3, 4 , Rebecca R. Pompano 1, 2, 3, 4, 5
Affiliation
|
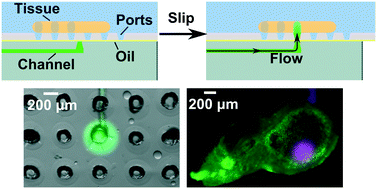
Many in vivo tissue responses begin locally, yet most in vitro stimuli are delivered globally. Microfluidics has a unique ability to provide focal stimulation to tissue samples with precise control over fluid location, flow rate, and composition. However, previous devices utilizing fixed ports beneath the tissue required manual alignment of the tissue over the ports, increasing the risk of mechanical damage. Here we present a novel microfluidic device that allows the user to define the location of fluid delivery to a living tissue slice without manipulating the tissue itself. The device utilized a two-component SlipChip design to create a mobile port beneath the tissue slice. A culture chamber perforated by an array of ports housed a tissue slice and was separated by a layer of fluorocarbon oil from a single delivery port, fed by a microfluidic channel in the movable layer below. We derived and validated a physical model, based on interfacial tension and flow resistance, to predict the conditions under which fluid delivery occurred without leakage into the gap between layers. Aqueous solution was delivered reproducibly to samples of tissue and gel, and the width of the delivery region was controlled primarily by convection. Tissue slice viability was not affected by stimulation on the device. As a proof-of-principle, we showed that live slices of lymph node tissue could be sequentially targeted for precise stimulation. In the future this device may serve as a platform to study the effects of fluid flow in tissues and to perform local drug screening.
中文翻译:

用户定义的通过可移动微流体端口对活组织的局部刺激†
许多体内组织反应从局部开始,但大多数体外刺激物在全球范围内传递。微流体具有独特的能力,可以通过精确控制流体的位置,流速和成分来对组织样本提供聚焦刺激。然而,利用在组织下方的固定端口的先前设备需要在端口上方手动对齐组织,从而增加了机械损坏的风险。在这里,我们介绍了一种新颖的微流控设备,它使用户可以定义向活体组织切片输送流体的位置,而无需操纵组织本身。该设备利用两组分滑动芯片设计在组织切片下方创建一个可移动端口。由一系列孔口穿孔的培养室中装有组织切片,并由一层碳氟化合物油从单个输送口中隔开,并由下方可移动层中的微流体通道供入。我们基于界面张力和流阻推导并验证了物理模型,以预测发生流体输送而不会渗入层间间隙的条件。将水溶液可再现地递送至组织和凝胶样品,并且递送区域的宽度主要通过对流来控制。组织切片的生存力不受设备刺激的影响。作为原理的证明,我们证明了淋巴结组织的活切片可以依次靶向以进行精确刺激。将来,该设备可以用作研究组织中流体流动的影响并进行局部药物筛选的平台。将水溶液可再现地递送至组织和凝胶样品,并且递送区域的宽度主要通过对流来控制。组织切片的生存力不受设备刺激的影响。作为原理的证明,我们证明了淋巴结组织的活切片可以依次靶向以进行精确刺激。将来,该设备可以用作研究组织中流体流动的影响并进行局部药物筛选的平台。将水溶液可再现地递送至组织和凝胶样品,并且递送区域的宽度主要通过对流来控制。组织切片的生存力不受设备刺激的影响。作为原理的证明,我们证明了淋巴结组织的活切片可以依次靶向以进行精确刺激。将来,该设备可以用作研究组织中流体流动的影响并进行局部药物筛选的平台。
更新日期:2018-06-15
中文翻译:

用户定义的通过可移动微流体端口对活组织的局部刺激†
许多体内组织反应从局部开始,但大多数体外刺激物在全球范围内传递。微流体具有独特的能力,可以通过精确控制流体的位置,流速和成分来对组织样本提供聚焦刺激。然而,利用在组织下方的固定端口的先前设备需要在端口上方手动对齐组织,从而增加了机械损坏的风险。在这里,我们介绍了一种新颖的微流控设备,它使用户可以定义向活体组织切片输送流体的位置,而无需操纵组织本身。该设备利用两组分滑动芯片设计在组织切片下方创建一个可移动端口。由一系列孔口穿孔的培养室中装有组织切片,并由一层碳氟化合物油从单个输送口中隔开,并由下方可移动层中的微流体通道供入。我们基于界面张力和流阻推导并验证了物理模型,以预测发生流体输送而不会渗入层间间隙的条件。将水溶液可再现地递送至组织和凝胶样品,并且递送区域的宽度主要通过对流来控制。组织切片的生存力不受设备刺激的影响。作为原理的证明,我们证明了淋巴结组织的活切片可以依次靶向以进行精确刺激。将来,该设备可以用作研究组织中流体流动的影响并进行局部药物筛选的平台。将水溶液可再现地递送至组织和凝胶样品,并且递送区域的宽度主要通过对流来控制。组织切片的生存力不受设备刺激的影响。作为原理的证明,我们证明了淋巴结组织的活切片可以依次靶向以进行精确刺激。将来,该设备可以用作研究组织中流体流动的影响并进行局部药物筛选的平台。将水溶液可再现地递送至组织和凝胶样品,并且递送区域的宽度主要通过对流来控制。组织切片的生存力不受设备刺激的影响。作为原理的证明,我们证明了淋巴结组织的活切片可以依次靶向以进行精确刺激。将来,该设备可以用作研究组织中流体流动的影响并进行局部药物筛选的平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号