Nature Reviews Drug Discovery ( IF 122.7 ) Pub Date : 2018-06-15 , DOI: 10.1038/nrd.2018.75 Patrick A. Mayes , Kenneth W. Hance , Axel Hoos
|
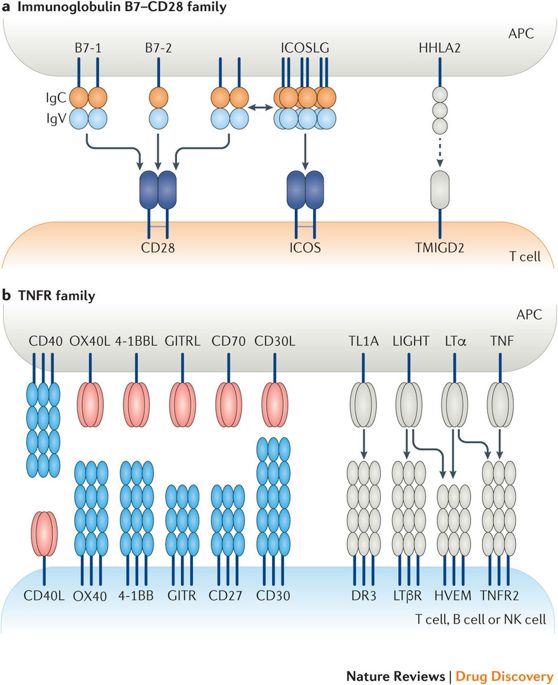
Immune cell functions are regulated by co-inhibitory and co-stimulatory receptors. The first two generations of cancer immunotherapy agents consist primarily of antagonist antibodies that block negative immune checkpoints, such as programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte protein 4 (CTLA4). Looking ahead, there is substantial promise in targeting co-stimulatory receptors with agonist antibodies, and a growing number of these agents are making their way through various stages of development. This Review discusses the key considerations and potential pitfalls of immune agonist antibody design and development, their differentiating features from antagonist antibodies and the landscape of agonist antibodies in clinical development for cancer treatment.
中文翻译:

癌症中免疫激动剂抗体开发的前景和挑战
免疫细胞功能受共同抑制和共同刺激受体的调节。前两代癌症免疫治疗剂主要由阻断阴性免疫检查点的拮抗剂抗体组成,例如程序性细胞死亡蛋白1(PD1)和细胞毒性T淋巴细胞蛋白4(CTLA4)。展望未来,用激动剂抗体靶向共刺激受体有很大的希望,并且越来越多的这类药物正在经历各个发展阶段。这篇综述讨论了免疫激动剂抗体设计和开发的关键考虑因素和潜在陷阱,它们与拮抗剂抗体的区别特征以及激动剂抗体在癌症治疗临床开发中的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号