Separation and Purification Technology ( IF 8.6 ) Pub Date : 2018-06-15 , DOI: 10.1016/j.seppur.2018.06.022 Yunfeng Song , Zhongwei Zhao
|
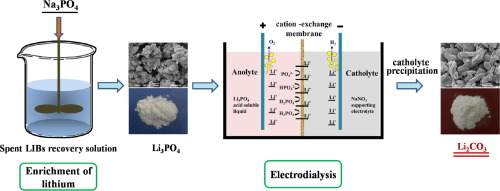
At present, the projected demand for lithium calls for processing all viable resources especially secondary resources. This paper presents a promising approach for recovering lithium from low lithium high-salt solution which is usually produced in the spent LIBs recycling process. The solution was firstly purified, and then lithium was precipitated by phosphate, the effects of operating conditions on the Li3PO4 precipitation behaviors were evaluated. The results indicated that temperature is a more important factor than seed crystal or flocculant. After that, Li3PO4 was dissolved by acid as the anolyte, and electrodialysis with cation-exchange membranes was used to investigate the separation performance of Li and P. The results showed that Li and P was effectively separated by electrodialysis, and the P/Li mass ratio of the catholyte was reduced to 0.23 (6.5 times lower compared to a feed P/Li ratio of 1.48). Deep separation of lithium and phosphorus in the catholyte was achieved by raising pH to make the lithium precipitated with the permeated phosphorus. The lithium concentration of purified catholyte was 22.5 g/L and was used to prepare lithium carbonate. The Li2CO3 precipitation rate reached 88.3% at 80 °C under CO33−/Li+ molar ratio of 1.1:2. The final product of lithium carbonate was in accord with the standard specification (Li2CO3-0, GB/T 11075-2013). The results of this study provide an efficient and green cyclic process for recovering lithium from spent LIBs.
中文翻译:

使用沉淀和电渗析技术从废锂离子电池中回收锂
当前,对锂的预计需求要求处理所有可行资源,特别是二次资源。本文提出了一种从低锂高盐溶液中回收锂的有前途的方法,这种方法通常是在废旧LIB回收过程中产生的。首先将溶液纯化,然后用磷酸盐使锂沉淀,评估操作条件对Li 3 PO 4沉淀行为的影响。结果表明,温度是比晶种或絮凝剂更重要的因素。之后,Li 3 PO 4用酸作为阳极电解液溶解,然后用阳离子交换膜进行电渗析研究Li和P的分离性能。结果表明,通过电渗析可以有效分离Li和P,并且阴极电解液的P / Li质量比降低到0.23(与进料P / Li比1.48相比降低了6.5倍)。通过提高pH值使锂与渗透的磷沉淀,可以实现阴极电解液中锂和磷的深度分离。纯化的阴极电解液的锂浓度为22.5 g / L,用于制备碳酸锂。在CO 3 3− / Li +下,在80°C下,Li 2 CO 3的沉淀率达到88.3%摩尔比为1.1:2。碳酸锂的最终产物符合标准规格(Li 2 CO 3 -0,GB / T 11075-2013)。这项研究的结果为从废LIB中回收锂提供了一种高效且绿色的循环过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号