Catalysis Communications ( IF 3.4 ) Pub Date : 2018-06-15 , DOI: 10.1016/j.catcom.2018.06.011 Jiena Yun , Chang Zhu , Qian Wang , Qiaoli Hu , Gang Yang
|
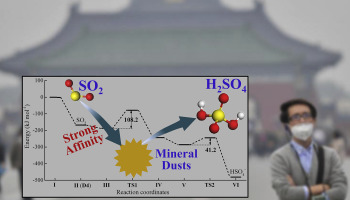
Mineral dust is an important arena for acid rain formation. Here, p-DFT calculations were conducted to study SO2 adsorption onto gibbsite and possible catalytic mechanisms for acid rain formation. The obtained results clearly indicated that SO2 is preferentially adsorbed onto mineral dusts instead of staying in the gas phase, and two catalytic paths were then posed. Path A with first hydrolysis and then oxidation is kinetically preferred, where SO2 hydrolysis appeared to be the rate-determining step for all the investigated surfaces. Partially dehydrated gibbsite (100) surface has the lowest reaction barrier, and a second water molecule causes acid rain formation to occur facilely at ambient circumstances.
中文翻译:

矿物粉尘对二氧化硫具有很强的亲和力,并且对酸雨形成具有催化作用
矿物粉尘是形成酸雨的重要场所。在这里,进行p-DFT计算以研究SO 2在三水铝石上的吸附以及酸雨形成的可能催化机理。所获得的结果清楚地表明,SO 2优先吸附在矿物粉尘上而不是停留在气相中,然后设置了两条催化路径。在动力学上优选先水解然后氧化的路径A,其中SO 2水解似乎是所有研究表面的决定速率的步骤。部分脱水的三水铝铁矿(100)表面具有最低的反应势垒,并且第二个水分子导致在周围环境中容易发生酸雨形成。










































 京公网安备 11010802027423号
京公网安备 11010802027423号