当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
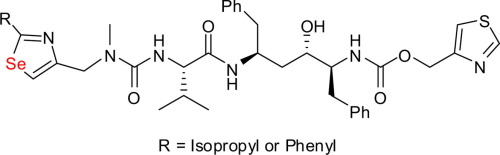
Design and synthesis of selenazole-substituted ritonavir analogs
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-06-15 , DOI: 10.1016/j.bmcl.2018.06.027 Junfei Qiao , Chuanfang Zhao , Jun Liu , Yuguo Du
中文翻译:

亚硒唑取代的利托那韦类似物的设计与合成
更新日期:2018-06-15
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-06-15 , DOI: 10.1016/j.bmcl.2018.06.027 Junfei Qiao , Chuanfang Zhao , Jun Liu , Yuguo Du
|
With the help of Surflex-Dock calculation, two ritonavir analogs in which one thioazole unit was replaced by selenazole have been designed and synthesized. The key selenazole structure was constructed from β-azido diselenide through a cascade diselenide cleavage/selenocarbonylation/Staudinger reduction/aza-Wittig reaction and a following MnO2 oxidation. The accordingly prepared compounds exhibited good anti-HIV-1 (IIIB) activities comparable to that of the original ritonavir, as well as the positive SI values.
中文翻译:

亚硒唑取代的利托那韦类似物的设计与合成
借助于Surflex-Dock计算,已设计并合成了两个利托那韦类似物,其中一个硫唑单元被硒仑唑代替。通过级联二硒化物裂解/硒代羰基化/施陶丁格还原/氮杂-维蒂希反应和随后的MnO 2氧化,由β-叠氮二硒化物构建关键的硒唑结构。相应制备的化合物显示出与原始利托那韦相当的良好的抗HIV-1(IIIB)活性,以及正的SI值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号